Glimepiride intermediate preparation method
An intermediate and ethyl technology, applied in the field of medicine, can solve the problems of long reaction time, highly toxic NaCN material, human and environmental hazards, etc., and achieve the effects of improving market competitiveness, reducing production costs, and reducing contact opportunities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of preparation method of glimepiride intermediate, such as figure 2 shown, including the following steps:
[0045] (1) Using ethyl acetoacetate as a raw material, in the presence of a strong base, sodium ethoxide, through bromoethane substitution reaction, to generate ethyl 2-ethyl-3-oxobutanoate;
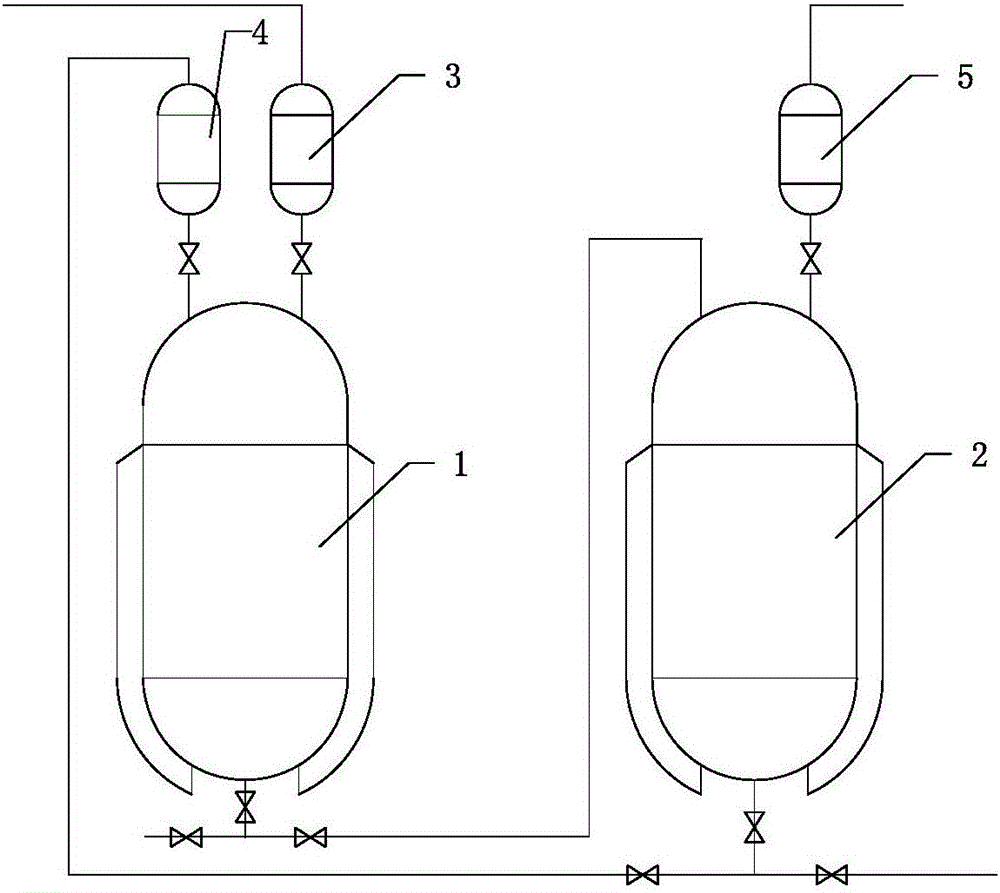
[0046] (2) 2-ethyl-3-oxobutanoic acid ethyl ester generates 2-ethyl-3-hydroxyl-3-cyanobutanoic acid ethyl ester through reaction with NaCN / sulfuric acid cyanation system in the cyanidation reactor, Such as figure 1 Shown, the special equipment that uses comprises cyanide reactor 1 and pickling tank 2, and the top of described cyanide reactor 1 is connected with NaCN metering tank 3 and sulfuric acid dropping tank 4; The top of described pickling tank 2 is connected There is an acid washing metering tank 5; and the bottom of the cyanidation reaction kettle 1 is connected to the top of the pickling kettle 2 by a pipeline, and the bottom of the pickling kettle 2 is co...
Embodiment 2
[0059] A preparation method of glimepiride intermediate, comprising the steps of:
[0060] (1) Using ethyl acetoacetate as a raw material, in the presence of a strong base, sodium ethoxide, through bromoethane substitution reaction, to generate ethyl 2-ethyl-3-oxobutanoate;
[0061] (2) 2-ethyl-3-oxobutanoic acid ethyl ester generates 2-ethyl-3-hydroxyl-3-cyanobutanoic acid ethyl ester through reaction with NaCN / sulfuric acid cyanation system in the cyanidation reactor, The special equipment that uses comprises cyanide reactor and pickling tank, and the top of described cyanide reactor is connected with NaCN metering tank and sulfuric acid dropping tank; The top of described pickling still is connected with washing acid metering tank; The lower part of the cyanide reaction kettle is connected to the top of the pickling kettle by a pipeline, and the bottom of the pickling kettle is connected back to the sulfuric acid dropping tank by a pipeline;
[0062] The detailed process i...
Embodiment 3
[0074] A preparation method of glimepiride intermediate, comprising the steps of:
[0075] (1) Using ethyl acetoacetate as a raw material, in the presence of a strong base, sodium ethoxide, through bromoethane substitution reaction, to generate ethyl 2-ethyl-3-oxobutanoate;
[0076] (2) 2-ethyl-3-oxobutanoic acid ethyl ester generates 2-ethyl-3-hydroxyl-3-cyanobutanoic acid ethyl ester through reaction with NaCN / sulfuric acid cyanation system in the cyanidation reactor, The special equipment that uses comprises cyanide reactor and pickling tank, and the top of described cyanide reactor is connected with NaCN metering tank and sulfuric acid dropping tank; The top of described pickling still is connected with washing acid metering tank; The lower part of the cyanide reaction kettle is connected to the top of the pickling kettle by a pipeline, and the bottom of the pickling kettle is connected back to the sulfuric acid dropping tank by a pipeline;
[0077] The detailed process i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com