Triazolone-thiadiazole compound capable of promoting regeneration of myocardial cells and drug use of triazolone-thiadiazole compound
A technology of thiadiazole derivatives and triadimefon, which is applied in the field of biomedicine, can solve the problems of unknown key targets, and achieve the effects of high activity, high druggability, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of CDNG1A and CDNG1B.
[0040] A two-step synthesis method is used:
[0041] Intermediate: 4-amino-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol(III) 4-amino-5-(pyridin-4-yl)-4H-1 ,24,-triazole-4-mercapto synthesis, refer to the literature JMC 2009, 52(14), 4200-4209 (including the synthesis method of compound 16):
[0042] Carbon disulfide (3.3ml, 5.5mmol) was added dropwise to a solution of KOH (3.0g, 5.5mmol) and pyridylisoniazid (3.6mmol) in ethanol (70ml). The mixture was stirred at room temperature overnight, then cooled in an ice-water bath and diluted with diethyl ether. The precipitate was filtered off, washed with diethyl ether and dried. The obtained product (1.6mmol) was dissolved in water (2ml), hydrazine hydrate (2.4mmol) was added, and the mixture was refluxed for 6hr under stirring. Then 80 ml of water was added to the reaction mixture and neutralized with concentrated hydrochloric acid, and the precipitate was filtered with water a...
Embodiment 2
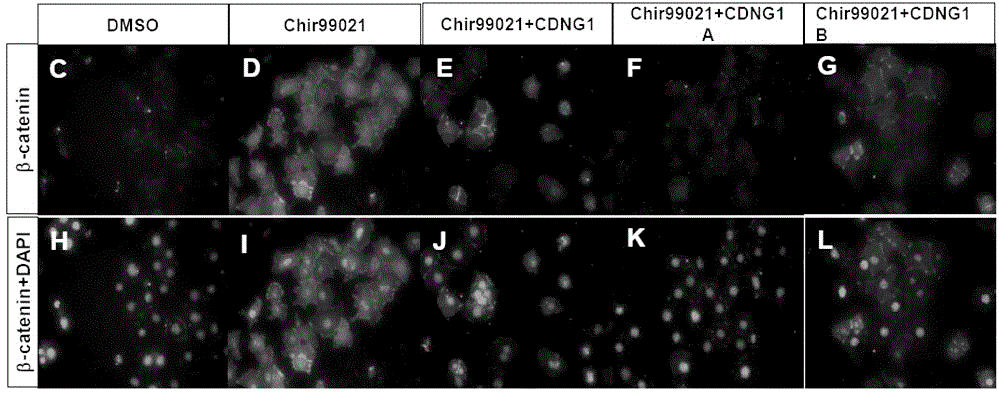
[0046] Example 2: Western blotting immunoblotting method to further verify that the effect of CDNGs on the Wnt pathway is achieved by inhibiting β-catenin ( figure 2 ).
[0047] step one
[0048] Cells: mouse CGR8 embryonic stem cells
[0049] Reagents: Known Wnt pathway agonist Chir99021, CDNG1 / CDNG1A / CDNG1B; DMEM (Sigma), 10% FBS, 100 units / ml LIF, 2 mM L-glutamine, 50 μM β-mercaptoethanol, Liposome 3000 (Life Tech), protease inhibitors, RIPA cell lysate, polyacrylamide gel, nitrocellulose membrane, skim milk powder, β-catenin primary antibody (Santa Cruz Biotechnology), GAPDH primary antibody (Santa Cruz Biotechnology), HRP secondary antibody.
[0050] Experimental procedure: The monolayer of mouse CGR8 embryonic stem cells was plated in serum-free DMEM, and 10% FBS, 100 units / ml LIF, 2mM L-glutamine and 50 μM β-mercaptoethanol were added, and placed at 37°C containing 5 %CO 2 cultured in a humid incubator. Cells were seeded into 24-well plates when they were subconfl...
Embodiment 3
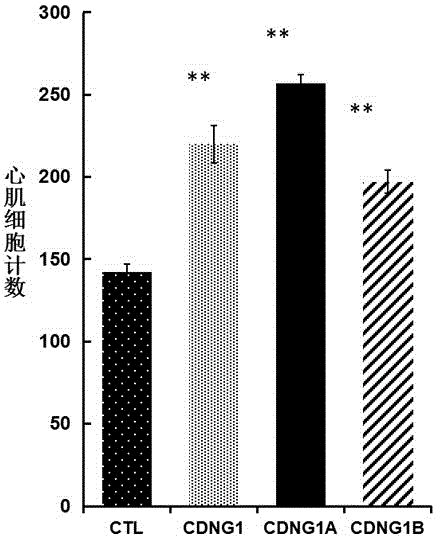
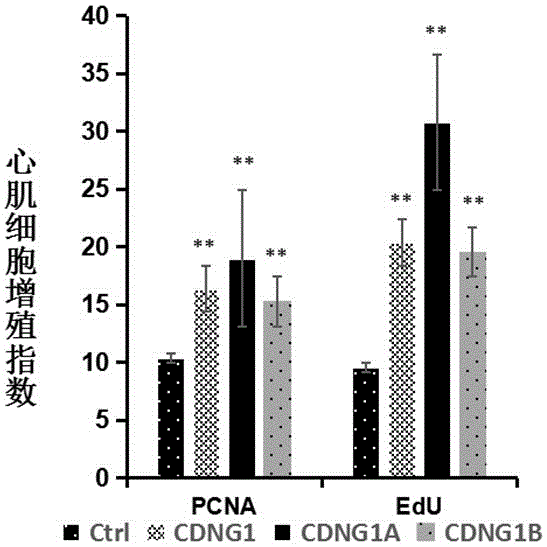
[0057] Example 3: Immunohistochemical analysis CDNG1A promotes the proliferation and regeneration of zebrafish cardiomyocytes.
[0058] Animals: 4-12 month old AB wild line and transgenic zebrafish Tg(cmlc2:nDsRed-nuc).
[0059] Reagents: Primary Antibody: Rabbit anti-Mef2c (Santa Cruz); Mouse anti-PCNA (Sigma); Click-iT® EdU Alexa-Fluor-488 Kit (Invirogen); Secondary Antibody Goat Anti-Mouse Alexa-Fluor-488 , Alexa-Fluor-555 goat anti-rabbit Alexa-Fluor-488, Alexa-Fluor-555 (both purchased from Invitrogen); Bouin's solution (prepared) containing 5% acetic acid, 9% formaldehyde, 0.9% picric acid (Sigma); Insoluble aniline blue dye (Fisher); G orange dye (Sigma); acid magenta dye.
[0060] Experimental procedure: Use surgical scissors to anesthetize 4-12-month-old zebrafish with anesthesia for 2 minutes, turn the belly of the fish upward under a stereomicroscope, and cut open the abdomen at the heart to expose the heart. Carefully and quickly cut off about 20% of the apex and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com