A kind of preparation method of nanometer cuprous oxide catalyst
A nano-cuprous oxide and cuprous oxide technology, which is applied in the direction of copper oxide/copper hydroxide, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problems of not being gentle and environmentally friendly, Achieve good photocatalytic performance, mild reaction conditions and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
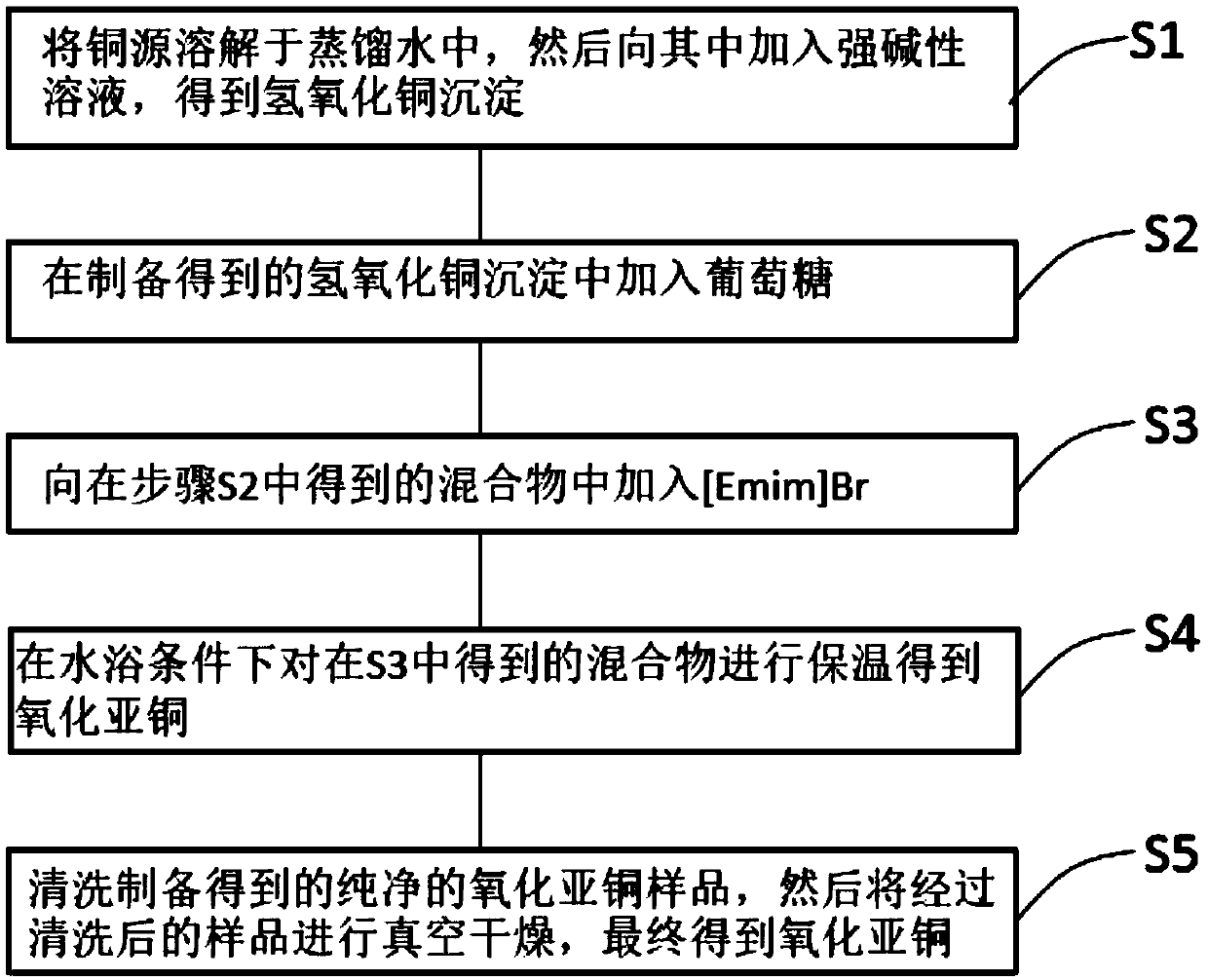
[0030] Such as figure 1 Shown, the preparation method of nano cuprous oxide catalyst provided by the invention comprises as follows:
[0031] S1, dissolving the copper source in distilled water, and then adding a strong alkaline solution to it to obtain copper hydroxide precipitation;
[0032] In the present invention, the strong alkaline solution is a sodium hydroxide solution, of course, the strong alkaline solution may also be a strong alkaline solution such as potassium hydroxide, which is not limited in the present invention. The copper source may be copper chloride, copper nitrate or copper sulfate, wherein the molar ratio of copper element to hydroxide is between 1:8-1:4.
[0033] S2, want to add glucose in the copper hydroxide precipitation that obtains in step S1;
[0034] Preferably, in the present invention, the glucose is D-(+)-glucose. Of course, the glucose may also be other types of glucose, which is not limited in the present invention.
[0035] S3, adding ...
Embodiment 1
[0049] S1. Dissolve copper chloride dihydrate in distilled water, and then add sodium hydroxide solution therein to obtain copper hydroxide precipitation; in this embodiment, the moles of copper and sodium (ie, copper and hydroxide) The ratio is 1:4,
[0050] S2, want to add D-(+)-glucose in the copper hydroxide precipitation obtained in step S1;
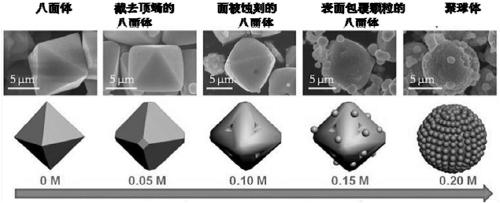
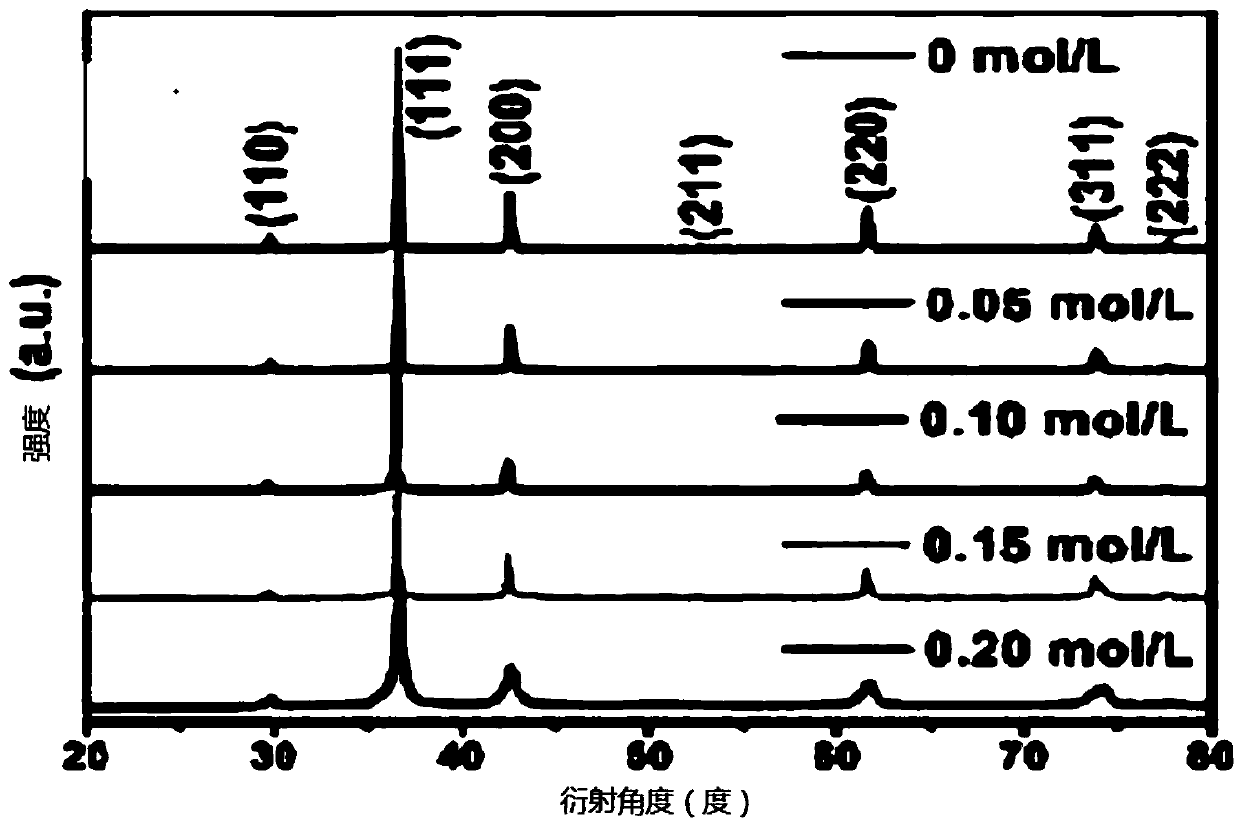
[0051] S3. Add [Emim]Br to the mixture obtained in step S2, wherein the [Emim]Br concentration of the mixture in step S3 is 0.05mol / L;
[0052] S4. Insulating the mixture obtained in step S3 under water bath conditions to obtain cuprous oxide;
[0053] In the present invention, a water bath is used to keep warm the mixture obtained in step S3, wherein the temperature of the water area is 60°C. The processing time was 20 minutes.
[0054] S5. Washing the prepared pure cuprous oxide several times with distilled water and alcohol respectively, and then vacuum-drying the cleaned sample to finally obtain cuprous oxide. Then, in a vac...
Embodiment 2
[0056] S1, dissolving copper chloride dihydrate in distilled water, and then adding sodium hydroxide solution thereto to obtain copper hydroxide precipitation; in this embodiment, the moles of copper and sodium (ie copper and hydroxide) The ratio is 1:6,
[0057] S2, want to add D-(+) glucose to the copper hydroxide precipitation obtained in step S1;
[0058] S3. Add [Emim]Br to the mixture obtained in step S2, wherein the [Emim]Br concentration of the mixture in step S3 is 0.10mol / L;
[0059] S4. Insulating the mixture obtained in step S3 under water bath conditions to obtain cuprous oxide;
[0060] In the present invention, a water bath is used to keep warm the mixture obtained in step S3, wherein the temperature of the water area is 50°C. The processing time was 30 minutes.
[0061] S5. Washing the prepared pure cuprous oxide several times with distilled water and alcohol respectively, and then vacuum-drying the cleaned sample to finally obtain cuprous oxide. Then, in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com