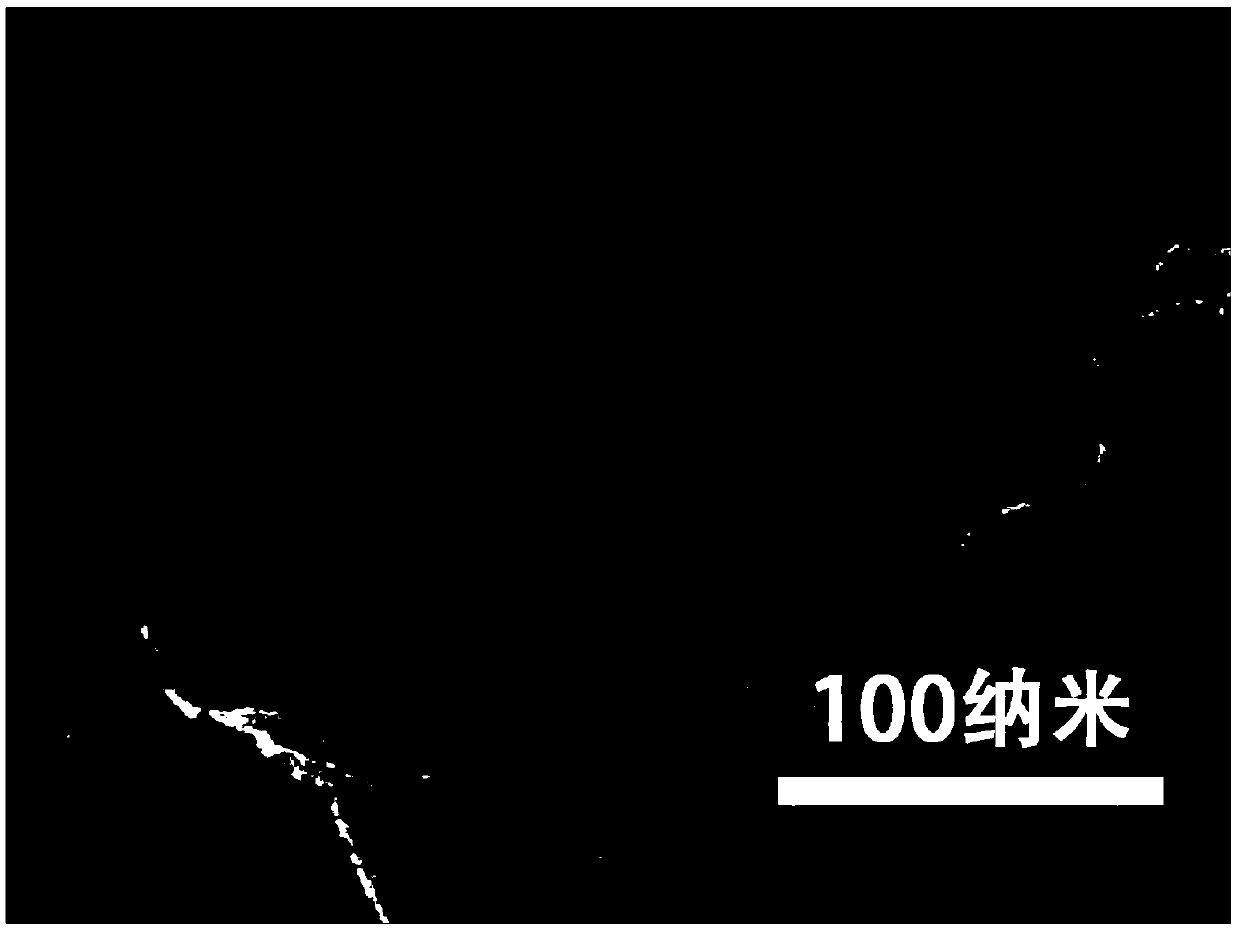
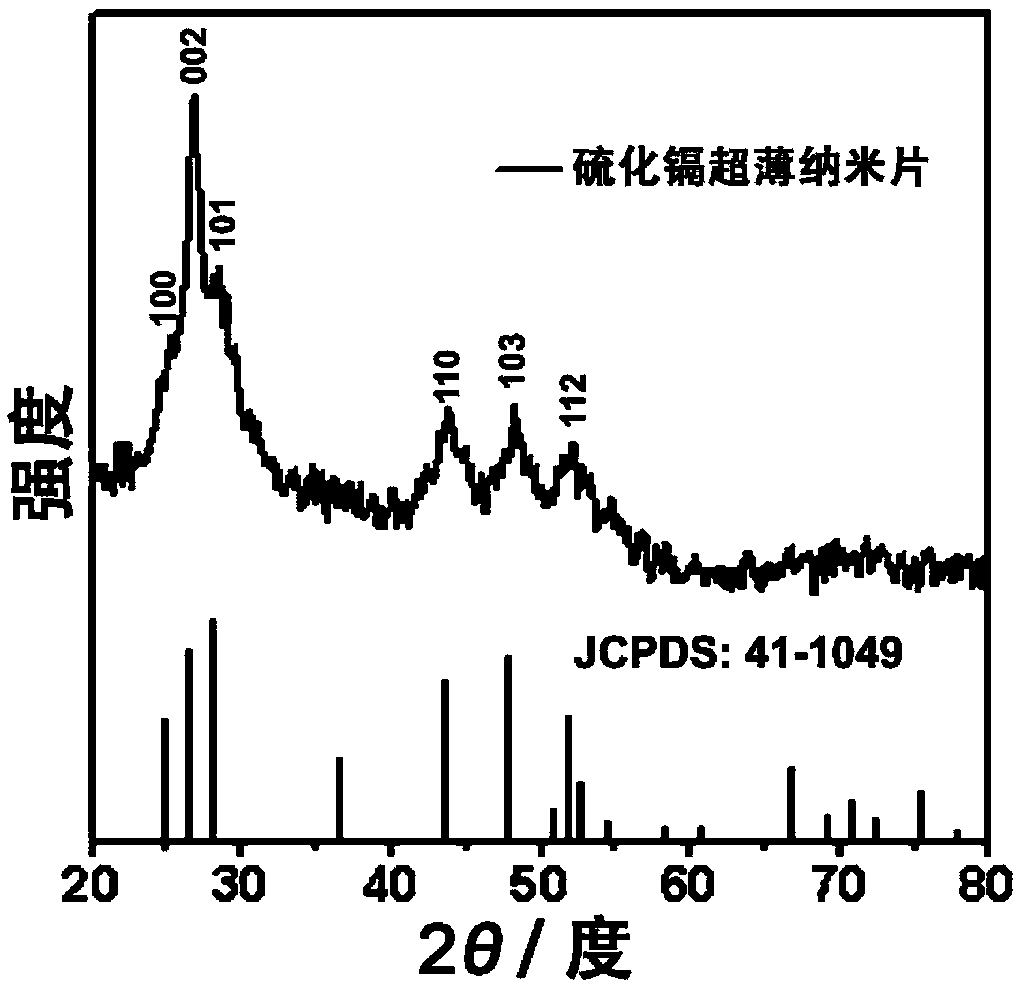
Cadmium sulfide ultrathin nanometer flaky material preparation method and application thereof
A nano-flaky, cadmium sulfide technology, applied in cadmium sulfide, chemical instruments and methods, nanotechnology, etc., can solve the problems of easy agglomeration, increased surface energy, and decreased activity of ultra-thin sheet-shaped materials, and achieves low-cost, synthetic Effect of low temperature and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Raw materials: analytically pure cadmium chloride 5 / 2 hydrate, sulfur powder, diethylenetriamine, L-cysteine.
[0039] 2. Weigh 0.0732g of cadmium chloride 5 / 2 hydrate and 0.064g of sulfur powder in a 20mL autoclave, and add 12mL of diethylenetriamine into it, and stir magnetically for 30 minutes at room temperature.
[0040] 3. Seal the autoclave well, put it in an oven and react at 70°C for 48 hours.
[0041] 4. After the reactor was naturally cooled to room temperature, it was taken out, and the product was transferred from the reactor to a centrifuge tube for centrifugation, washed with water and ethanol three times, and then dried in a vacuum oven at 40°C for 6 hours.
[0042] 5. Weigh 20 mg of the above product and 10 mg of L-cysteine into a 100 mL beaker, then add 0.1 mL of diethylenetriamine and 50 mL of water, and then put the beaker into an ultrasonic machine for continuous low-power ultrasonication for two hours.
[0043] 6. Transfer the sonicated produ...
Embodiment 2
[0045] 1. Raw materials: analytically pure cadmium chloride 5 / 2 hydrate, sulfur powder, diethylenetriamine, L-cysteine.
[0046] 2. Weigh 0.183g of cadmium chloride 5 / 2 hydrate and 0.16g of sulfur powder into a 50mL autoclave, and add 30mL of diethylenetriamine into it, and stir magnetically for 30 minutes at room temperature.
[0047] 3. Seal the autoclave well, put it in an oven and react at 80°C for 48 hours.
[0048] 4. After the reactor was naturally cooled to room temperature, it was taken out, and the product was transferred from the reactor to a centrifuge tube for centrifugation, washed with water and ethanol three times, and then dried in a vacuum oven at 40°C for 6 hours.
[0049] 5. Weigh 75mg of the above product and 25mg of L-cysteine into a 250mL beaker, then add 0.25mL of diethylenetriamine and 100mL of water to it, and then put the beaker into an ultrasonic machine for continuous low-power ultrasonication for two hours.
[0050] 6. Transfer the sonicated pr...
Embodiment 3
[0052] 1. Raw materials: analytically pure cadmium chloride 5 / 2 hydrate, sulfur powder, diethylenetriamine, L-cysteine.
[0053] 2. Weigh 0.366g of cadmium chloride 5 / 2 hydrate and 0.32g of sulfur powder in a 100mL autoclave, and add 60mL of diethylenetriamine into it, and stir magnetically for 30 minutes at room temperature.
[0054] 3. Seal the autoclave well, put it in an oven and react at 90°C for 40 hours.
[0055] 4. After the reactor was naturally cooled to room temperature, it was taken out, and the product was transferred from the reactor to a centrifuge tube for centrifugation, washed with water and ethanol three times, and then dried in a vacuum oven at 40°C for 6 hours.
[0056] 5. Weigh 750mg of the above product and 50mg of L-cysteine into a 500mL beaker, then add 0.5mL of diethylenetriamine and 150mL of water to it, and then put the beaker into an ultrasonic machine for continuous low-power ultrasonication for two hours.
[0057] 6. Transfer the sonicated pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com