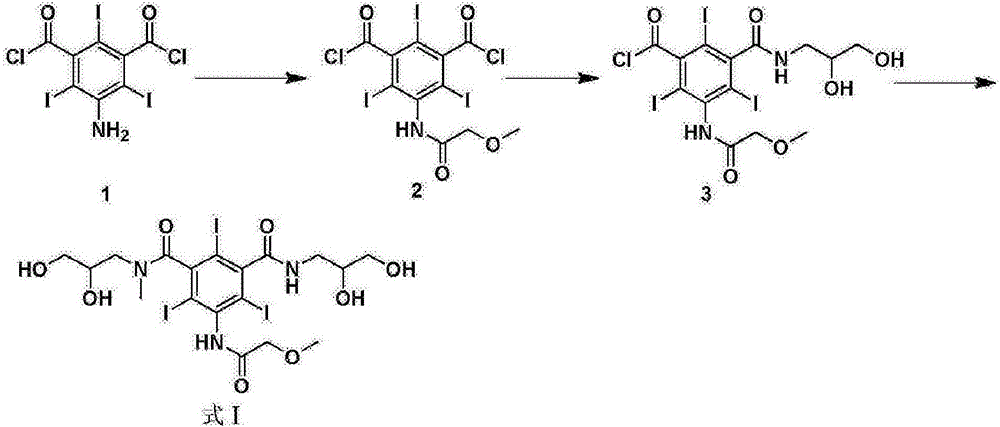
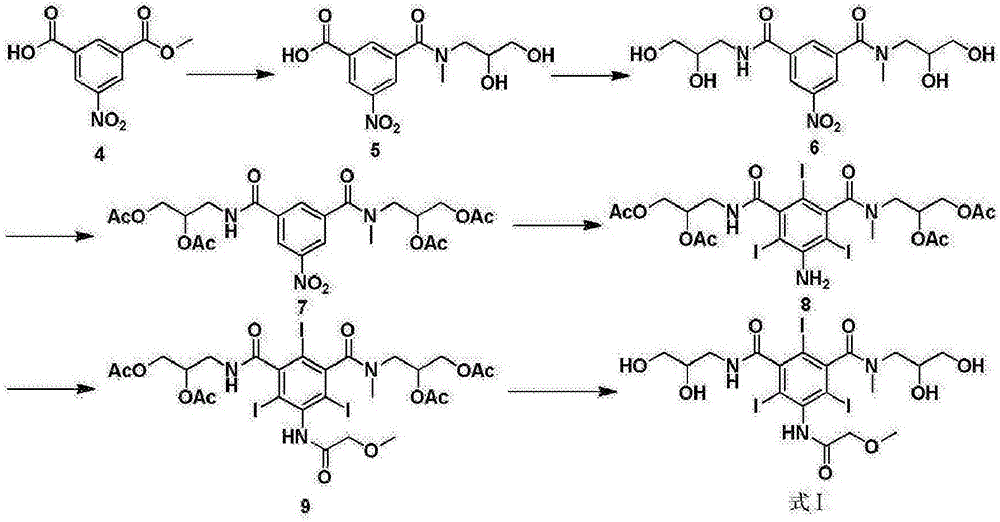
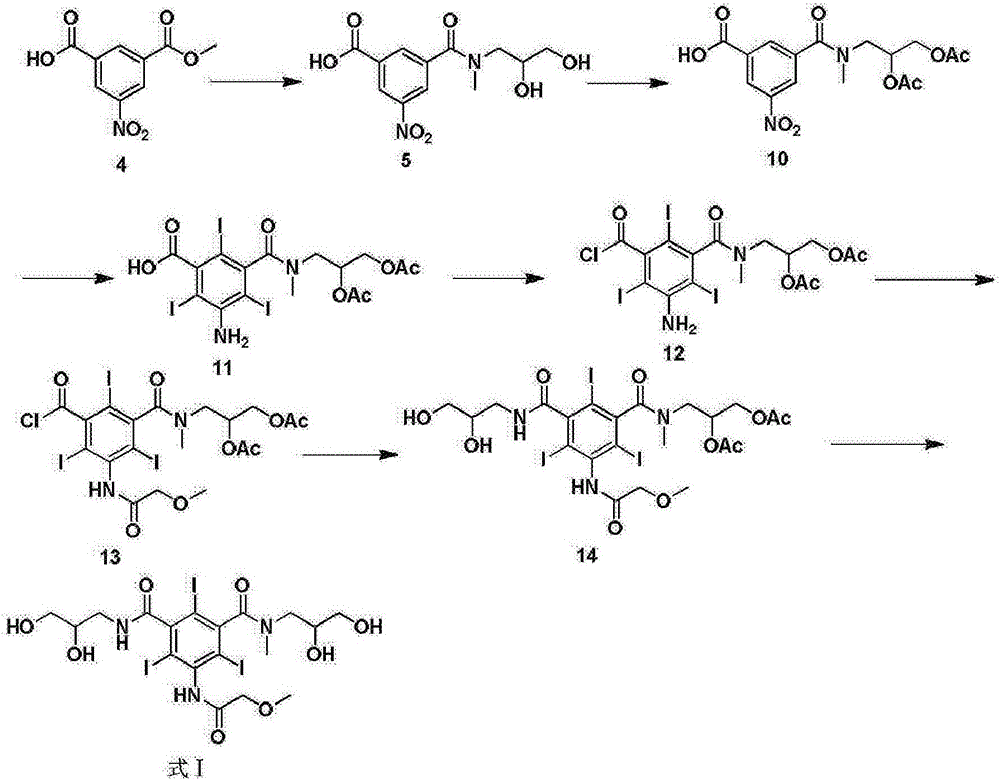
Preparation method of iopromide
A technology of iopromide and amidomethyl ester, which is applied in the field of preparation of iopromide, can solve problems such as difficult acquisition of raw materials, difficulty in purchasing, and difficulty in purification, so as to avoid the introduction of ionic compounds or salts, avoid large water consumption, and avoid Effect of desalination operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (S1) Sulfuric acid catalyzed preparation of 5-amino-2,4,6-triiodoisophthalic acid methyl ester (formula 4)
[0045] Add 22.5kg of 5-amino-2,4,6-triiodoisophthalic acid and 120kg of methanol into a 200L reactor, slowly add 200g of sulfuric acid, turn on steam and heat to reflux, stir, and react for 4 hours. The liquid phase monitors the complete conversion of raw materials. Concentrate under reduced pressure to recover methanol. Methanol was recovered, cooled to 30°C, poured into 160kg of ice water, continued to cool down to 10°C, kept warm and crystallized for 2 hours, centrifuged to obtain off-white solid, dried to obtain 22.3kg, yield 95%, HPLC: 99.2%.
[0046] (S2) Preparation of 5-methoxyacetamido-2,4,6-triiodoisophthalamide (formula 3)
[0047] Add 17.6kg of formula 4 and 2.5kg of methoxyacetyl chloride into the reactor containing 80kg of dioxane, raise the temperature to reflux, stir, and react for 4 hours. High-efficiency liquid phase monitors that the convers...
Embodiment 2
[0053] (S1) Methanesulfonic acid catalyzed preparation of 5-amino-2,4,6-triiodoisophthalic acid methyl ester (formula 4)
[0054] Add 22.5kg of 5-amino-2,4,6-triiodoisophthalic acid and 120kg of methanol into a 200L reactor, slowly add 196g of methanesulfonic acid, turn on steam and heat to reflux, stir, and react for 4 hours. The liquid phase monitors the complete conversion of raw materials. Concentrate under reduced pressure to recover methanol. Methanol was recovered, cooled to 30°C, poured into 160kg of ice water, continued to cool down to 10°C, kept warm and crystallized for 2 hours, centrifuged to obtain off-white solid, dried to obtain 22.5kg, yield 95.9%, HPLC: 99.5%.
[0055] (S2) Preparation of 5-methoxyacetamido-2,4,6-triiodoisophthalamide (formula 3)
[0056] Add 17.6kg of formula 4 and 2.5kg of methoxyacetyl chloride into the reactor containing 80kg of dioxane, raise the temperature to reflux, stir, and react for 4 hours. High-efficiency liquid phase monitors ...
Embodiment 3
[0062] (S1) catalyzed preparation of 5-amino-2,4,6-triiodoisophthalic acid methyl ester by p-toluenesulfonic acid (Formula 4)
[0063] Add 22.5kg of 5-amino-2,4,6-triiodoisophthalic acid and 120kg of methanol into a 200L reactor, slowly add 305g of p-toluenesulfonic acid, turn on the steam, heat to reflux, stir, and react for 4 hours. The liquid phase monitors the complete conversion of raw materials. Concentrate under reduced pressure to recover methanol. Methanol was recovered, cooled to 30°C, poured into 160kg of ice water, continued to cool down to 10°C, kept warm and crystallized for 2 hours, centrifuged to obtain off-white solid, dried to obtain 22.2kg, yield 94.6%, HPLC: 99.5%.
[0064] (S2) Preparation of 5-methoxyacetamido-2,4,6-triiodoisophthalamide (formula 3)
[0065] Add 17.6kg of formula 4 and 2.5kg of methoxyacetyl chloride into the reactor containing 80kg of dioxane, raise the temperature to reflux, stir, and react for 4 hours. High-efficiency liquid phase m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com