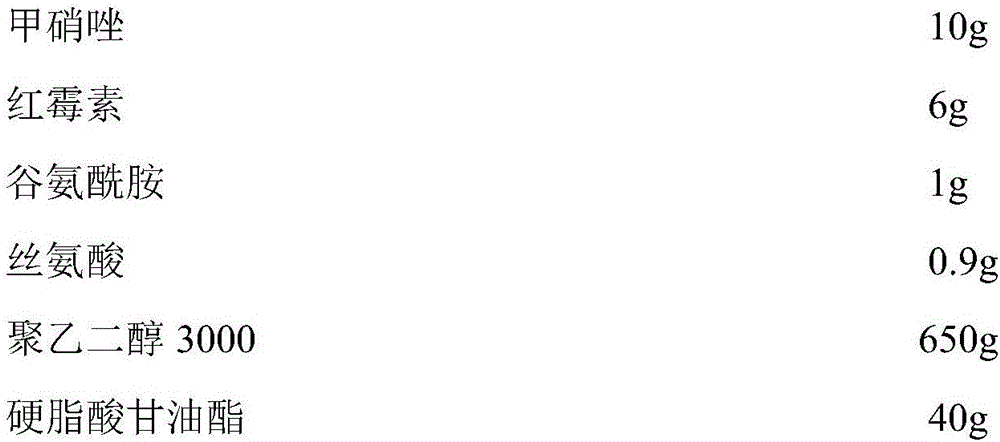Metronidazole-erythromycin compound ointment for treating face acnes and preparation method thereof
A technology for metronidazole erythromycin and facial acne, applied in the field of medicine, can solve problems such as affecting facial appearance, easy recurrence of erythromycin, pigmentation and the like, and achieve the effects of good effectiveness, good stability and improved effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Prescription composition:
[0026]
[0027] Preparation:
[0028] (1) Heat polyethylene glycol 1500 to 70°C to melt, add 1 / 3 of the formula amount of water for injection, stir and mix to obtain the mixed solution I, and keep the constant temperature at 70°C for later use;
[0029] (2) Add carboxymethylcellulose sodium, stearyl alcohol and sodium hyaluronate to 2 / 3 of the formula amount of water for injection, stir and mix to obtain mixed solution II;
[0030] (3) Add metronidazole, erythromycin, glutamine and serine into the mixed solution II, stir and mix to obtain the mixed solution III;
[0031] (4) Heat the mixed solution III in a water bath to 70°C, then add the mixed solution I to the mixed solution III, stir in the same direction to fully emulsify, and cool naturally to obtain the product.
Embodiment 2
[0033] Prescription composition:
[0034]
[0035]
[0036] Preparation:
[0037] (1) Heat polyethylene glycol 3000 to 80°C to melt, add 1 / 3 of the formula amount of water for injection, stir and mix well to obtain mixed solution I, and keep the temperature at 80°C for later use;
[0038] (2) Add carboxymethylcellulose sodium, glyceryl stearate and sodium hyaluronate to 2 / 3 of the formula amount of water for injection, stir and mix to obtain mixed solution II;
[0039] (3) Add metronidazole, erythromycin, glutamine and serine into the mixed solution II, stir and mix to obtain the mixed solution III;
[0040] (4) Heat the mixed solution III in a water bath to 80°C, then add the mixed solution I to the mixed solution III, stir in the same direction to fully emulsify, and cool naturally to obtain the product.
Embodiment 3
[0042] Prescription composition:
[0043]
[0044] Preparation:
[0045] (1) Heat polyethylene glycol 4000 to 70°C to melt, add 1 / 3 of the formula amount of water for injection, stir and mix to obtain the mixed solution I, and keep the constant temperature at 70°C for later use;
[0046] (2) Add sodium carboxymethylcellulose, sodium lauryl sulfate and sodium hyaluronate to 2 / 3 of the formula amount of water for injection, stir and mix to obtain mixed solution II;
[0047] (3) Add metronidazole, erythromycin, glutamine and serine into the mixed solution II, stir and mix to obtain the mixed solution III;
[0048] (4) Heat the mixed solution III in a water bath to 70°C, then add the mixed solution I to the mixed solution III, stir in the same direction to fully emulsify, and cool naturally to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com