Monopotassium phosphate production method
A technology of potassium dihydrogen phosphate and calcium hydrogen phosphate, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of low utilization rate of raw materials and high production cost, so as to improve utilization rate, low cost and reduce production. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
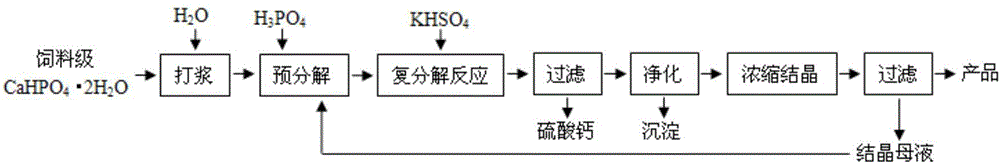
[0059] Mix 315kg of feed grade calcium hydrogen phosphate and 787.5kg of water in the reactor evenly, add 174.43kg of phosphoric acid solution with a mass percentage of 20%, the reaction temperature is 50°C, and add 727.07kg of 30% by mass of sulfuric acid after reacting for 1h Potassium hydrogen solution, the reaction temperature is 50°C, and the reaction time is 2h; after the reaction is completed, filter to obtain calcium sulfate and potassium dihydrogen phosphate-containing filtrate, and the loss rates of phosphorus and potassium in calcium sulfate are 4.93% and 0.26% respectively; After the filtrate was purified, it was filtered to remove impurities, and the resulting filtrate was concentrated and crystallized. During the crystallization process, the stirring speed was 80rpm / min, and the crystallization temperature was 20°C. After stirring and crystallizing for 3 hours, 136.01kg of potassium dihydrogen phosphate product and 494.81kg of mother liquor were obtained by filtrat...
Embodiment 2
[0061] Mix 230kg of feed grade calcium hydrogen phosphate and 1150kg of water in the reactor evenly, add 95.52kg of phosphoric acid solution with a mass percentage of 40%, the reaction temperature is 70°C, and add 389.31kg of 50% by mass of sulfuric acid after 2 hours of reaction Potassium hydrogen solution, the reaction temperature is 70°C, and the reaction time is 4h; filter after the reaction to obtain calcium sulfate and filtrate, the loss rates of phosphorus and potassium in calcium sulfate are 4.89% and 0.34% respectively; after the filtrate is purified, filter to remove Impurities, the obtained filtrate is concentrated and crystallized, the stirring speed of the crystallization process is 100rpm / min, and the crystallization temperature is 40°C. After stirring and crystallizing for 6h, filter to obtain 32.90kg of potassium dihydrogen phosphate product and 545.44kg of mother liquor, and the mother liquor is returned to the reactor. Circulation operation is carried out; the...
Embodiment 3
[0063] Mix 180kg of feed grade calcium hydrogen phosphate and 647.50kg of water in the reactor, add 426.87kg of mother liquor, and the reaction temperature is 70°C. After 1 hour of reaction, add 304.68kg of potassium hydrogensulfate solution with a mass percentage of 50%. The temperature is 70°C, and the reaction time is 4h; after the reaction is finished, filter to obtain calcium sulfate and filtrate, and the loss rates of phosphorus and potassium in calcium sulfate are 4.91% and 0.42% respectively; after the filtrate is purified, filter to remove impurities, and the obtained filtrate is processed Concentrated crystallization, the stirring speed of the crystallization process is 100rpm / min, and the crystallization temperature is 40°C. After stirring and crystallizing for 6 hours, 114.01kg of potassium dihydrogen phosphate product and 477.91kg of mother liquor are obtained by filtration, and the mother liquor is returned to the reactor for recycling operation; the product The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com