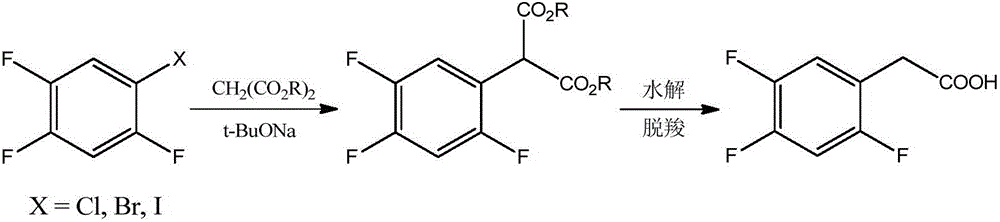
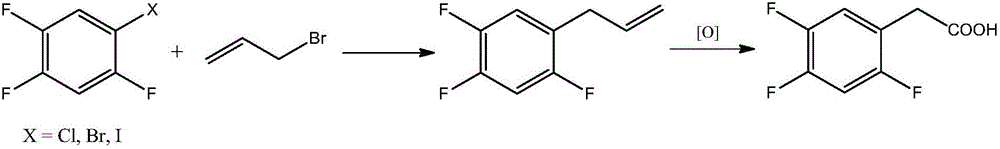
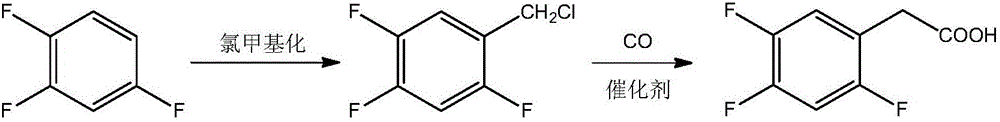
Novel method for preparing 2,4,5-trifluoro phenylacetic acid
A technology of trifluorophenylacetic acid and trifluorophenylethyl alcohol, applied in the field of medicine and chemical industry, can solve the problems of low cost and hidden dangers, and achieve the effects of low cost, less discharge of three wastes, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 2.8g AlCl 3 Dissolve in 100g of 1,2-dichloroethane, heat to reflux, add 15g of 1,2,4-trifluorobenzene dropwise into the reaction system under vigorous stirring, continue to react at reflux temperature for 6 hours, cool and filter, The filtrate was distilled under reduced pressure at a temperature not higher than 50°C to recover unreacted 1,2-dichloroethane, and the remaining oil was directly used for the next reaction without separation.
Embodiment 2
[0039] 4.1g BF 3 ·C 2 h 5 OC 2 h 5 Dissolve in 100g 1,2-dichloroethane, heat to reflux, add 15g 1,2,4-trifluorobenzene dropwise into the reaction system under vigorous stirring, continue to react at reflux temperature for 8 hours, cool and filter , the filtrate was distilled under reduced pressure at a temperature not higher than 50°C to recover unreacted 1,2-dichloroethane, and the remaining oil was directly used for the next reaction without separation.
Embodiment 3
[0041] The oily product reacted in Example 1 was added to 150ml of distilled water, 4.0g of NaOH was added, and stirred at 30°C for 6 hours. After the reaction, the reaction system was extracted three times with 200ml of dichloromethane, and the organic layer was separated. Wash with water with pH=3 (100ml×3), dry with magnesium sulfate, filter, distill dichloromethane under normal pressure and recover, the remaining 17.8 grams of light yellow oil is 2,4,5-trifluorophenethyl alcohol The purity of the crude product determined by HPLC is 92%, and the two-step yield is 89%. This crude product does not need to be separated and can be directly carried out in the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com