Amphipathic tadpole-shaped block copolymer and preparation method thereof
A block copolymer and amphiphilic technology, which is applied in the field of amphiphilic tadpole-shaped block copolymer and its preparation, can solve the problems such as no research report on amphiphilic tadpole-shaped block copolymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
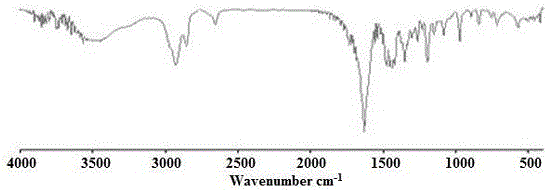
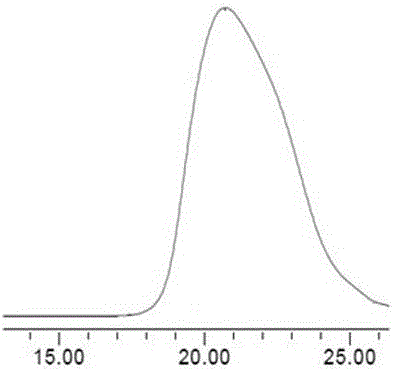
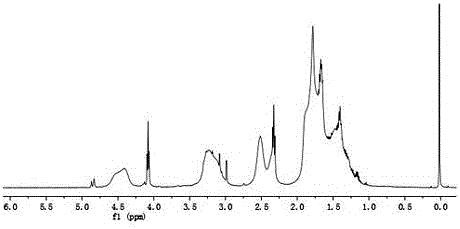
[0088] Add 0.28 g of ethylene glycol, 29.2 μL of stannous octoate, and 20 mL of ε-caprolactone (ε-CL) into a dry Schlenk flask, vacuumize and fill with nitrogen for several times, then place it in an oil bath at 100°C and stir for 14 hours. After cooling, 10 mL of ethanol was added, and the crude product was obtained by filtration. The crude product was dissolved in a small amount of THF, and co-precipitated three times with n-hexane to obtain polycaprolactone (HO-PCL-OH) with α, ω-double-terminal hydroxyl groups.
[0089] Take 4.5g HO-PCL-OH into a three-necked bottle, add 50mL CH 2 Cl 2 and 1.77mL triethylamine, in an ice-water bath, add 5mL of CH dissolved in 1.06mL 2-bromopropionyl bromide (BPB) 2 Cl 2 The solution was dripped and reacted at room temperature for 48h. Filter, add 100mLCH to the filtrate 2 Cl 2 After dilution, it was washed successively with 1.0M HCl (3×50 mL), 1.0M NaOH (3×50 mL), and 1.0M NaCl (3×50 mL) solutions, respectively. Anhydrous MgSO for or...
Embodiment 2
[0092] Take 0.42g X-PCL-X into a dry reaction bottle, add 5mL CH 2 Cl 2 make it dissolve, N 2 Under protection, 14.6 mg of n-butylamine and a small amount of tributylphosphine were added, and the reaction was stirred for 2 h. After the reaction, co-precipitate with petroleum ether 3 times. The precipitate was collected and dried at room temperature to obtain polycaprolactone (HS-PCL-SH) with α,ω-double-terminal mercapto groups, with a yield of 70%.
Embodiment 3
[0094] N 2 Under protection, 1.13g DTCA, 1.0g DCC were dissolved in 40mL CH 2 Cl 2, placed in an ice-water bath, slowly dropwise added 20 mL of CH with 0.5 g of propynyl alcohol and 60 mg of DMAP 2 Cl 2 After the solution was dropped, it was reacted in an ice-water bath for 1 h, and at room temperature for 24 h. After filtration, the filtrate was concentrated, and the residue was dissolved in ether, filtered, and the ether was distilled off to obtain a crude product. The crude product was separated by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =20:1) A thiocarbamate chain transfer agent (alknyl-CTA) with a terminal alkynyl group was obtained with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com