Antigen of epidemic encephalitis live vaccine and preparation method and application thereof
A technology of Japanese encephalitis and Japanese encephalitis virus, applied in the field of antigens and vaccines containing it, can solve the problems of difficult control of exogenous viruses, many miscellaneous proteins, and large differences between batches, so as to prevent Japanese encephalitis Effects of virus infection, good immunogenicity, and small batch-to-batch variation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
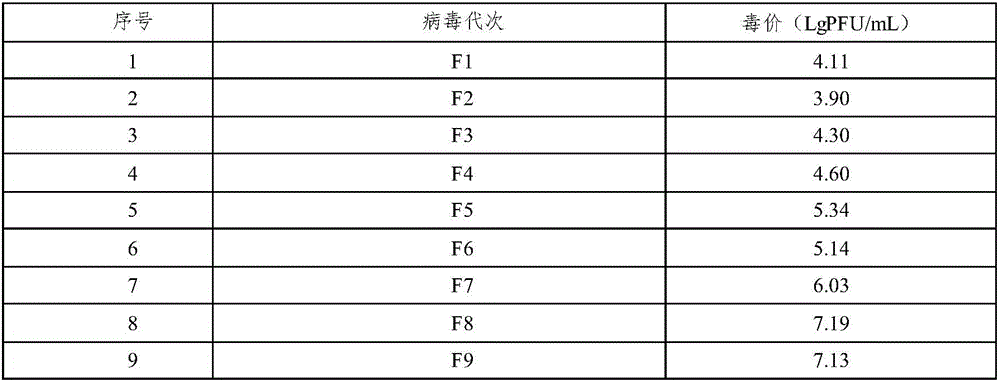
[0024] Example 1 Preparation and immunogenicity research of Japanese encephalitis virus attenuated strain adapted to Vero cells
[0025] (1) Adaptive passage of virus
[0026] Prepare Vero cell suspension with DMEM cell culture medium containing 8% calf serum, inoculate the culture flask according to the split ratio of 1:3, 37°C, 5% CO 2 Cultivate until the cells form a dense monolayer (about 48 hours in culture) for inoculation. Pour out the cell culture medium in the culture bottle before inoculation, wash it with PBS of pH 7.4 for 3 times, control the residual liquid after the last wash, and then inoculate attenuated Japanese encephalitis virus according to 5% of the culture medium volume strain (SA14-14-2). Adsorb at 37°C for 90 minutes after inoculation. After the adsorption is completed, DMEM maintenance solution containing 2% calf serum is added and incubated at 35°C. The cytopathic condition was observed daily, and the culture was terminated when the cytopathic rat...
Embodiment 2
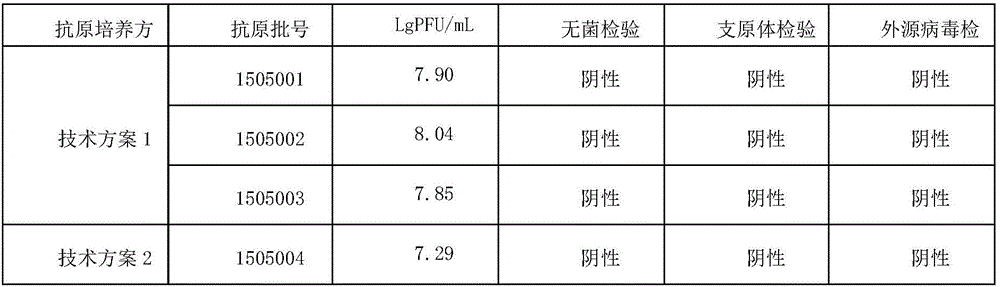
[0039] Embodiment 2 adopts 3L rotating bottle to prepare Japanese encephalitis live vaccine virus liquid (antigen)
[0040] (1) Antigen preparation
[0041]Technical scheme 1: adopt 3L spinner bottle to cultivate Vero, culture medium is the DMEM cell culture medium that contains 8% calf serum, treat that Vero cell forms monolayer, discard cell culture medium, wash 3 times with the PBS of pH7.4, discard Remove the PBS washing solution; inoculate the F8 Japanese encephalitis virus attenuated strain (SA14-14-2 strain) adapted to Vero cells at 0.2MOI (PFU / cell number), the rotation speed of the bottle machine is 12 rpm, 37°C Adsorb for 1 hour; add 0.22ul membrane filter to sterilize pH7.6 DMEM solution (containing 20mL / L calf serum, 10g / L arginine hydrochloride, 5g / L PEG2000 and 5g / L 4-hydroxyethyl Piperazineethanesulfonic acid), cultured at a constant temperature at 35°C, and the rotation speed of the bottle spinner was 12 rpm; about 3 to 4 days, 75% of the cells had lesions, an...
Embodiment 3
[0051] Embodiment 3 adopts 15L rotating bottle to prepare swine Japanese encephalitis live vaccine
[0052] (1) Antigen preparation
[0053] The Vero cells were cultivated in a 15L spinner bottle, and the culture medium was DMEM cell culture medium containing 8% calf serum. After the Vero cells formed a monolayer, the cell culture medium was discarded, and the PBS with pH 7.4 was used to wash 3 times, and the PBS was discarded and washed. solution; attenuated Japanese encephalitis virus strain of the F8 generation (SA14-14-2 strain) inoculated with Vero cells at 0.02 MOI (PFU / cell number), the rotation speed of the spinner was 20 revolutions / hour, and adsorbed at 37°C for 1h; Virus culture maintenance solution DMEM (containing 20 mL / L calf serum, 10 g / L arginine hydrochloride, 5 g / L PEG2000 and 5 g / L 4-hydroxyethylpiperazineethanesulfonic acid) at pH 7.8, 35 Cultivate at a constant temperature under the condition of ℃, and the rotation speed of the spinner is 20 rpm; when 75%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com