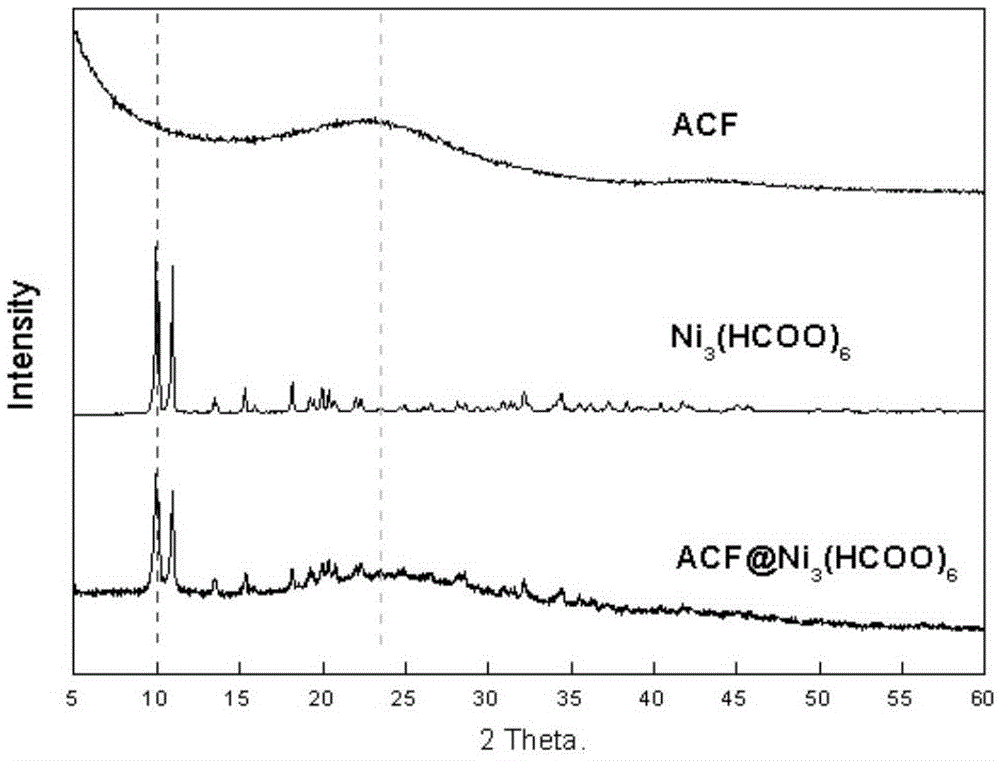
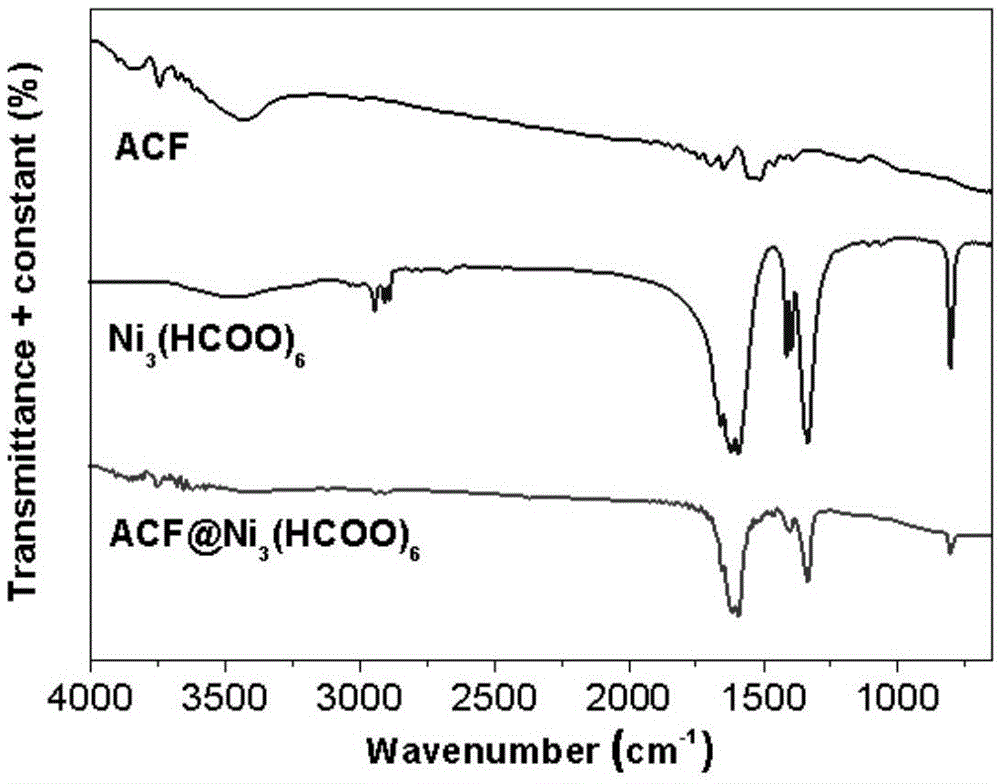
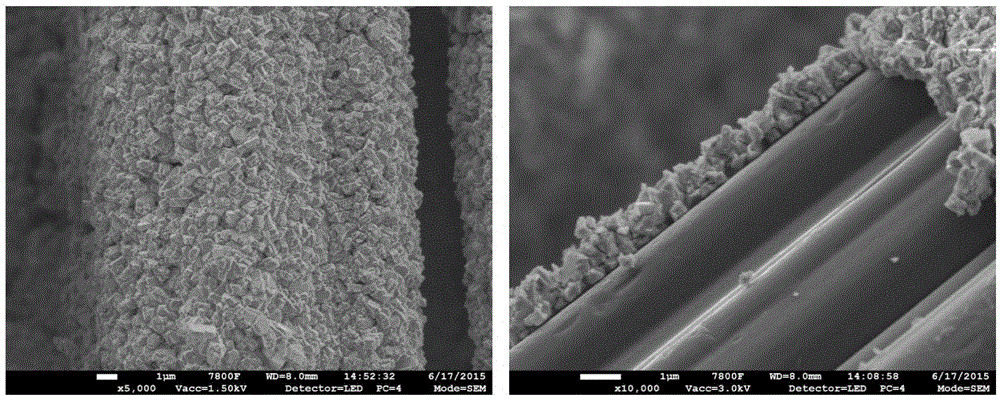
Preparation of active carbon fiber-metal organic framework composite material, and composite material and application
A metal-organic framework, activated carbon fiber technology, applied in non-metallic elements, fuels, inorganic chemistry, etc., can solve the problems of easy structure collapse, loss of performance, poor water resistance, heat resistance and stability, etc., and achieve high surface area, large pores, etc. Content, the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Preparation of activated carbon fiber felt@Al-BDC composite material
[0071] Use hydrochloric acid, nitric acid mixed acid (1:1) with pH = 2 to impregnate the activated carbon fiber felt three times, the impregnation volume is 1g / 20ml, 1g / 20ml, 1g / 100ml respectively, the impregnation time is 2h, 2h, 24h respectively, the acid is recycled and reused ; After the impregnation, the ACF was washed with distilled water to neutrality, then dried at 110°C for 12 hours, and set aside; weighed 1.88g Al(NO 3 ) 3 9H 2 O, 1.5g of urea in 50ml of water, stirred for 20min until evenly mixed, transferred to a 120ml autoclave, then weighed ca. ℃ for 12 hours; cool down naturally, take out the ACF complex, wash it twice with 20ml of water, and then dry it at 80℃ for 6h; weigh 3.88g of terephthalic acid (H 2 BDC) in 40ml DMF, stirred for 20min until evenly mixed, transferred to an autoclave, then vertically inserted the above-mentioned activated carbon fiber-metal precipita...
Embodiment 2
[0075] Example 2: Preparation of activated carbon fiber felt@CuBTC composite material
[0076] Process activated carbon fiber felt according to embodiment 1; Take by weighing 1.21g Cu(NO 3 ) 2 ·3H 2 O, 1.5g of urea in 50ml of water, stirred for 20min until evenly mixed, transferred to a 120ml autoclave, then weighed ca. ℃ for 12 hours; cool down naturally, take out the ACF complex, wash it twice with 20ml of water, and then dry it at 80℃ for 6h; weigh 2.035g of trimesic acid (H 3 BTC) in the mixed solution of 15ml water and 30ml ethanol, stir 20min to mix uniformly, transfer to the autoclave, then vertically insert the above-mentioned activated carbon fiber-metal precipitate precursor into the solution, and the reaction kettle is placed in an oven for 110 React at ℃ for 12h; cool down naturally, take out the ACF composite, then wash with 20ml ethanol for 3 times, dry at 80℃ for 4h, and then dry and activate at 150℃ and vacuum (0.2bar) for 12h to prepare activated carbon fib...
Embodiment 3
[0080] Example 3: Preparation of activated carbon fiber cloth @Cu(bdc)(dabco) 0.5 composite material
[0081] Process activated carbon fiber cloth according to embodiment 1; Take by weighing 1.21g Cu(NO 3 ) 2 ·3H 2 O, 1.5g of urea in 50ml of water, stirred for 20min until evenly mixed, transferred to a 120ml autoclave, then weighed ca. ℃ for 12 hours; cool down naturally, take out the ACF complex, wash it twice with 20ml of water, and then dry it at 80℃ for 6 hours; weigh 2.1g of terephthalic acid (H 2BDC) and 0.71g triethylenediamine (DABCO) were stirred in 40ml DMF for 20min to mix evenly, then transferred to the autoclave, then the above-mentioned activated carbon fiber-metal precipitate precursor was vertically inserted into the solution, and the reaction kettle was left standing React in a rotary oven at 110°C for 48h; cool down naturally, take out the ACF complex, then wash twice with 20ml DMF and diethyl ether, dry at 70°C for 6h, and then dry and activate at 160°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com