Tetraphenylmethylene chlorin compound, and preparation method and application thereof
A technology of tetraphenylmethylene chlorin and compound, which is applied in the field of tetraphenylmethylene chlorin and its preparation, and can solve the problems of poor stability, difficult synthesis, easy oxidation of Temoporfin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
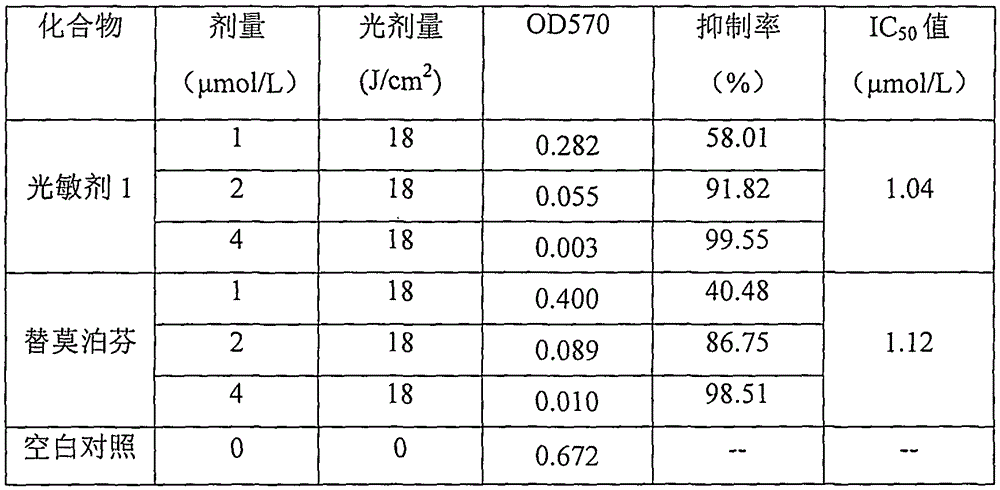
Examples
Embodiment 1
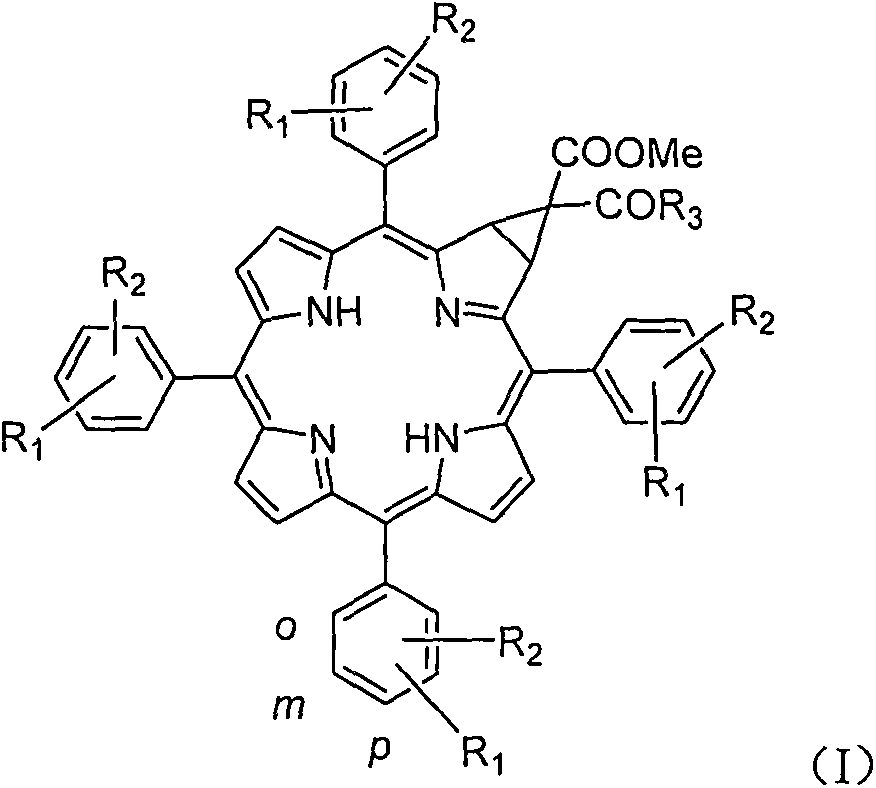
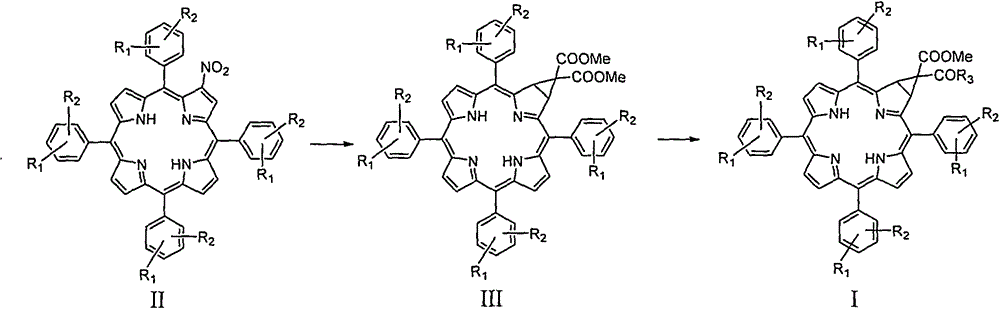
[0042] 1. Preparation method of 5,10,15,20-tetraphenyl-[2:3]-[(methoxycarbonyl, carboxyl)-methylene]chlorphine
[0043] In a 100mL three-necked flask, add potassium carbonate (620mg, 4.5mmol), dimethyl malonate (0.64mL, 6.8mmol) and 20mL dimethyl sulfoxide, and raise the temperature to 60°C under a nitrogen atmosphere and stir for about 1h; Then 2-nitro-5,10,15,20-tetraphenylporphyrin (400mg, 0.606mmol) was added and heated to 100°C for about 8 hours. The reaction was monitored by TLC until the reaction was complete. After the reaction solution was cooled to room temperature, 100 mL of water was added, the aqueous phase was extracted with dichloromethane (100 mL×3), and the organic phases were combined. The organic phase was washed with saturated brine (100 mL×3), dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure, and the resulting residue was subjected to column chromatography (eluent: dichloromethane:petroleum ether=1:10...
Embodiment 2
[0068] 1. Preparation method of 5,10,15,20-tetraphenyl-[2:3]-{[methoxycarbonyl, (2-hydroxyethyl)carbamoyl]-methylene}chlorphine
[0069] The synthesis of 5,10,15,20-tetraphenyl-[2:3]-[bis(methoxycarbonyl)-methylene]chlorin refers to Example 1.
[0070] In a 100mL three-necked flask, add 5,10,15,20-tetraphenyl-[2:3]-[bis(methoxycarbonyl)-methylene]chlorin (372mg, 0.5mmol), then Add 20mL tetrahydrofuran and 10mL ethanolamine, heat to reflux, and monitor by thin layer chromatography until the reaction is complete. After the reaction solution was cooled to room temperature, 150 mL of dichloromethane was added for extraction. The organic phase was washed with water (100 mL×3), washed with saturated brine (100 mL×3), dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure, and the resulting residue was subjected to column chromatography (eluent: dichloromethane:methanol=10:1) to obtain a purple solid powder 5,10,15,20-tetraphenyl-[2:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com