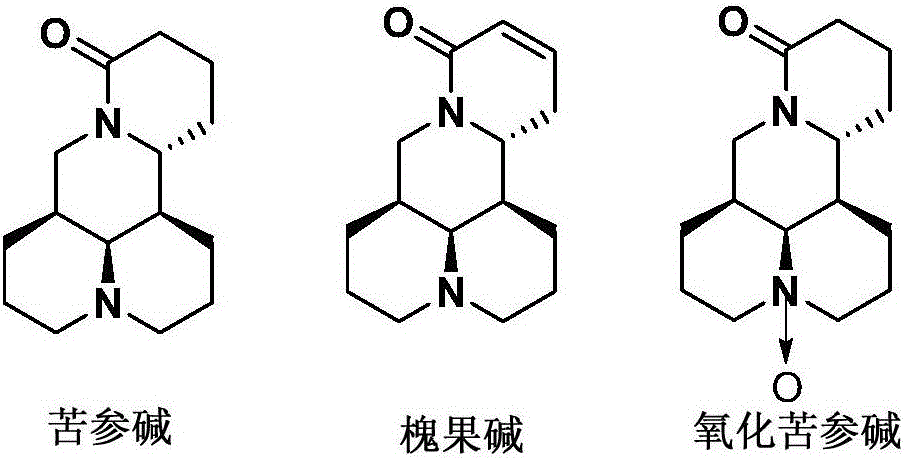
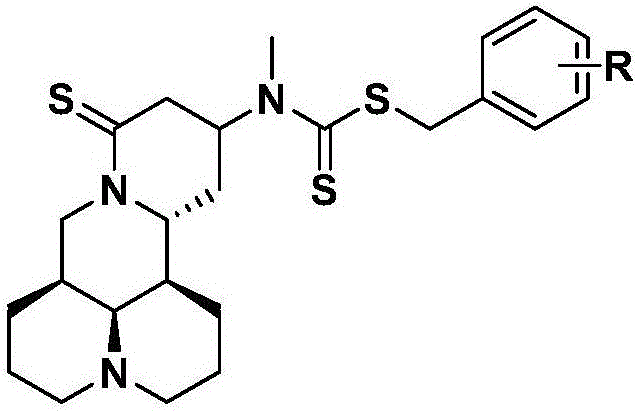
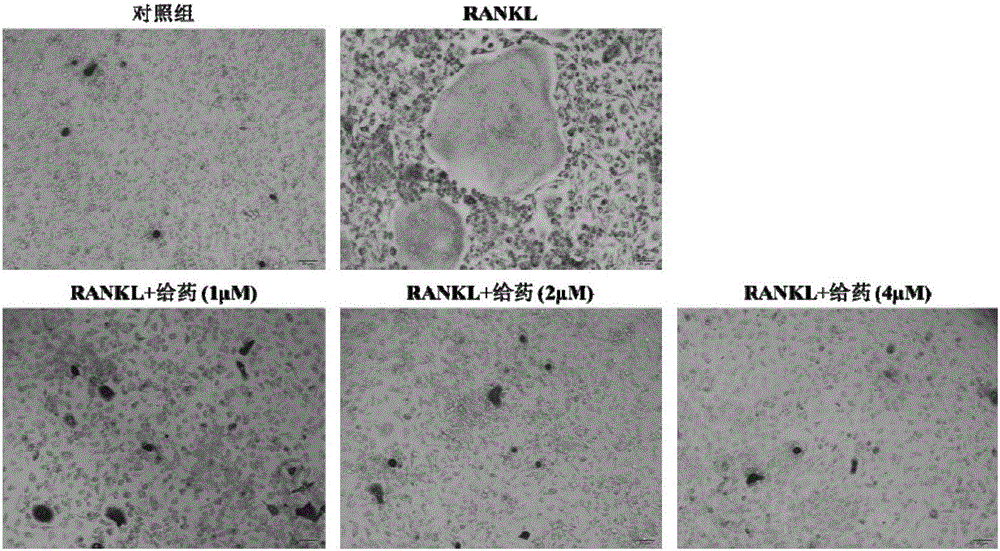
Application of group of thio-matrine derivative and salts thereof to preparation of anti-osteoporosis medicament
A technology of thiomatrine and anti-osteoporosis, which is applied in drug combination, bone disease, and pharmaceutical formula, can solve the problems of unreported effects, achieve high bioavailability, good stability, and reduce loss Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Preparation of 15-thiosophocarpine
[0033] 10.0 g (0.04 mol) of sophocarpine and 20.0 g (0.05 mol, purchased from Alfa Company) of sophocarpine were placed in a 500 ml reaction bottle, 200 ml of toluene was added, and the reaction was heated under reflux for 12 hours. After the reaction was completed, the solvent was concentrated under reduced pressure to remove the solvent, and the crude product was passed through a silica gel column with dichloromethane:methanol (25:1) as the eluent to obtain 9.6 g of the product with a yield of 91.2%.
Embodiment 2
[0034] Example 2: Preparation of 13-(N-methyl)-amino-15-thiomatrine (M19)
[0035] 10 g (4 mol) of 18-thiosophocarpine was placed in a 500 ml reaction bottle, 200 ml of methylamino alcohol solution and 10 ml of triethylamine were added, and the reaction was stirred for 12 hours. After the reaction was completed, the solvent was removed by concentration under reduced pressure, and the crude product was passed through a silica gel column with dichloromethane:methanol (20:1) as the eluent to obtain 9.8 g of the product with a yield of 83.5%.
Embodiment 3
[0036] Example 3: Preparation of 13-(N-methyl, N-difluorobenzyl dithioformate)-amino-18-thiomatrine (ZSM-1 in the table)
[0037] Put 240mg (0.8mmol) of 18-thiomatrine (M19) and 160mg (2mmol) of carbon disulfide in a 25ml reaction bottle, add 5ml of acetonitrile, add 374mg (2mmol) of p-fluorobenzyl bromide under stirring, and react at room temperature for 4 hours , After the reaction was completed, the organic solvent was distilled off under reduced pressure, and the crude product was passed through a silica gel column, and the eluent was dichloromethane: acetone (10:1) to obtain 354 mg of a white solid, with a yield of 92.5%.
[0038] The H NMR spectrum and mass spectrometry data of the product are:
[0039] 1 H-NMR (300MHz, CDCl 3 )δ7.41–7.29(m,2H),7.09–6.90(m,2H),6.10(s,1H),5.37(dd,J=12.0,3.8Hz,1H),4.49(d,J=14.5Hz ,3H),3.64–2.95(m,6H),2.84(s,2H),2.37–1.13(m,19H).
[0040] 13 C-NMR (75MHz, CDCl 3 )δ163.87,160.60,131.39,131.34,131.09,130.98,115.67,115.38,77.47,77.25,77....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com