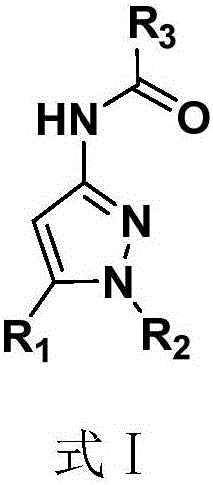
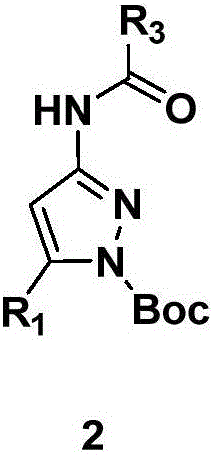
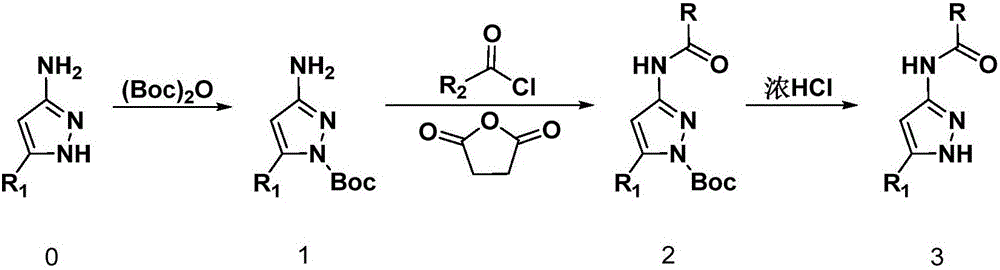
Pyrazole amide compounds as well as preparation method application of pyrazole amide compounds
A compound and pyrazole technology, applied in the field of pyrazole amide compounds and their preparation, can solve the problems of high cost of synthesis of brassinolide, many metabolic sites, easy metabolic inactivation and the like, so as to improve the ability of resisting lodging, Easy to prepare, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1, 4-(1H-pyrazole-3-amino)-4-oxobutanoic acid methyl ester (R in formula (I) 1 and R 2 is H, R is the synthesis of methyl butyrate)
[0056]
[0057] 1) Synthesis of N-Boc-3-aminopyrazole
[0058]
[0059] In a 100 mL round bottom flask, add 3-aminopyrazole (10 mmol) and di-tert-butyl dicarbonate (Boc) 2 O (14mmol), 15mL tetrahydrofuran (solvent A) was stirred and dissolved, added DMAP (0.6mmol, 6%) in an ice-salt bath and stirred for 2-4 hours, returned to room temperature, concentrated, beaten with ethyl acetate / petroleum ether, and filtered to obtain pure product. The specific method of solvent beating is: to 1g of the initial product to be purified, add the mixed solvent (V 乙酸乙酯 / V 石油醚 =2:1) 5mL, stirred at room temperature until the product was completely precipitated, and the pure product was obtained by suction filtration.
[0060] 2) Synthesis of 4-[(N-Boc-pyrazole)-3-amino]-4-oxobutanoic acid methyl ester
[0061]
[0062]In a 100mL ro...
Embodiment 2
[0070] Embodiment 2, the determination of the growth regulation activity of plant
[0071] (1) Hypocotyl elongation activity experiment of Arabidopsis mutant det2-1
[0072] Seeds of the Arabidopsis mutant det2-1 were sterilized with 70% ethanol for 1 min, and 1% sodium hypochlorite for 15 min at the same time, washed with sterile water and sown in 1 / 2MS (0.8% agar, 1% sucrose and compounds with specified concentrations); 4°C refrigerator After vernalization for 3 days, they were transferred to dark conditions and cultured at 22°C for 7 days. After the whole plant was photographed, the length of the hypocotyl was measured by ImageJ software.
[0073] test results
[0074] The test results of all compounds are shown in Tables 2 and 3.
[0075] Table 2 The test results of hypocotyl elongation of control drug Arabidopsis thaliana
[0076]
[0077] Table 3 The elongation test results of hypocotyls in Arabidopsis thaliana
[0078]
[0079] It can be seen from the above Ta...
Embodiment 3
[0080] Embodiment 3, paddy rice blade inclination experiment
[0081] Rice (Nipponbare) seeds use 10% H 2 o 2 Disinfect for 20 minutes, wash with sterile water, germinate in an incubator at 30°C for 3 days, and culture the young shoots under light for 5-6 days, cut off the leaf angle at 28°C and place it in different concentrations of medicinal liquid for 48 hours, measure with a protractor Leaf inclination.
[0082] test results
[0083] The test results of all compounds are shown in Tables 4 and 5 below
[0084] Table 4 Effects of control chemicals on the tilt angle of rice leaves
[0085]
[0086] Table 5 Effects of synthetic compounds on rice leaf tilt angle
[0087]
[0088] It can be seen from the above Tables 4 and 5 that 24-epiBL has a very high activity. At a concentration of 10 μM, the leaf tilt angle reaches 125±9°. However, when Bikinin is at a maximum concentration of 100 μM, its activity is not as good as that of 24-epiBL, which can only reach 75°. ±8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com