Synthesis technology of nitrophenol and intermediate thereof
A technology for nitrophenol and dinitrophenol, which is applied in the field of preparation of organic compounds, can solve the problems of substandard occupational hygiene, low safety, and large pollution, and achieves the effects of strong serial operability and avoiding large pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
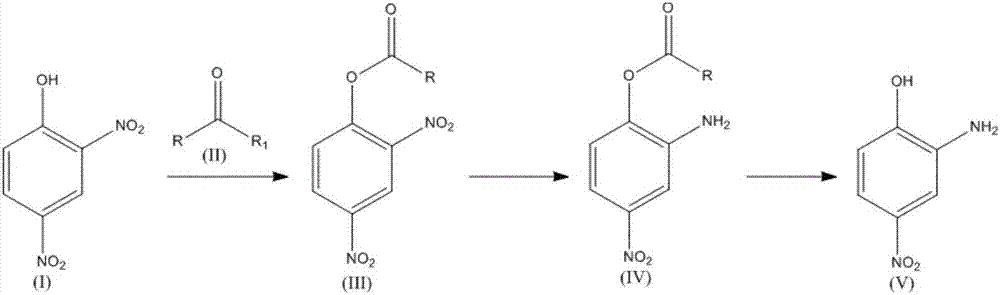
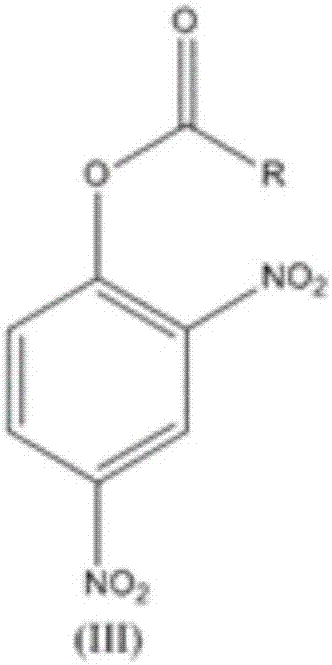
[0028] 184kg of 2,4-dinitrophenol was mixed with 79kg of acetyl chloride, 101kg of triethylamine, and 540kg of chloroform, and the reaction was completed to obtain 225kg of phenyl 2,4-dinitroacetate;
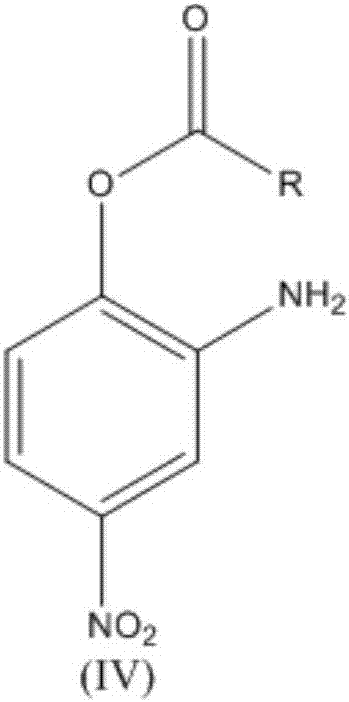
[0029] Mix 225kg 2,4-dinitroacetic acid phenyl ester with 450kg methanol, 0.67kg Pd / C, pass through hydrogen, 2,4-dinitroacetic acid phenyl ester is completely converted, and 181.5kg 2-amino-4 nitro Phenyl acetate;
[0030] By mixing 181.5 kg of 2-amino-4-nitrophenyl acetate with 185.2 kg of sodium hydroxide solution, the reaction was completed to obtain 140.6 kg of 2-amino-4-nitrophenol with a purity of 99.8%.
Embodiment 2
[0032] 184kg 2,4-dinitrophenol was mixed with 123kg isovaleryl chloride, 71.9kg potassium carbonate, and 368kg acetone, and the reaction was completed to obtain 266kg 2,4-dinitroisovalerate phenyl;
[0033] 266kg 2,4-dinitroisovalerate phenyl ester, 1064kg propyl acetate, 2.66kg Ru / C were mixed, the reaction was complete, and 207.9kg of 2-amino-4-nitroisovalerate phenyl ester with a purity of 98.7% was obtained .
Embodiment 3
[0035] By mixing 184kg 2,4-dinitrophenol with 123kg isovaleryl chloride, 71.9kg potassium carbonate, and 552kg dichloroethane, the reaction is completed to obtain 800kg 2,4-dinitroisovalerate phenyl dichloroethane solution ;
[0036] Through 800kg 2,4-dinitroisovalerate phenyl dichloroethane solution is mixed with 1.06kg Pd / C, stir until reaction is complete, obtain 2-amino-4-nitroisovalerate benzene of 221kg purity 99.7% ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com