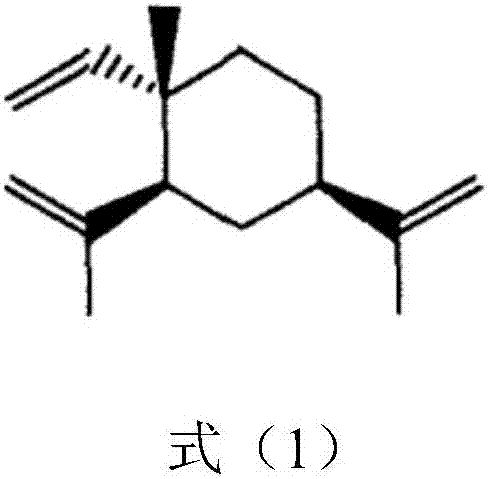
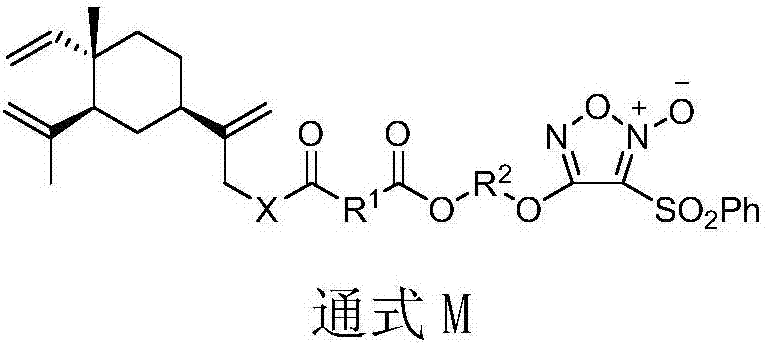
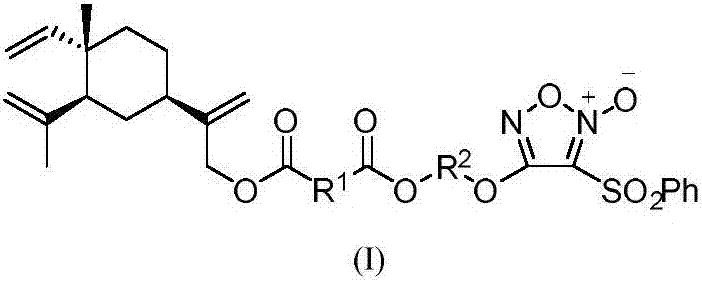
Nitric oxide donor type beta-elemene derivative and preparation method and application thereof
A technology of nitric oxide and elemene, applied in the direction of organic chemistry, drug combination, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] Preparation of intermediate 13-β-elemenol
[0074] Dissolve 100mmol of β-elemene in 20mL of dichloromethane and acetic acid mixed solution (V:V=2:1), and slowly drop into sodium hypochlorite solution containing 180mmol of active chlorine under ice-bath conditions, react in ice-bath for 4h and then separate the liquids. The aqueous layer was extracted 3 times with dichloromethane, and the combined dichloromethane was concentrated to obtain a light yellow liquid crude product. The liquid crude product was dissolved in 15 mL of anhydrous N,N-dimethylformamide (DMF), and 200 mmol of anhydrous sodium acetate was added under stirring. , and reacted at 100°C for 7 hours; the reaction liquid was filtered with 200 mesh silica gel, 15 mL of saturated saline was added to the filtrate, and then extracted three times with petroleum ether, combined and concentrated petroleum ether to obtain a yellow liquid, mixed solution of 8 mL of methanol and 8 mL of chloroform Dissolve the yellow...
Embodiment 1
[0079]
[0080] Preparation method of 13-O-(4-oxobutanoic acid-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxy)ethyl ester)-β-elemene as follows:
[0081] 1) Preparation of 3,4-diphenylsulfonyl-1,2,5-oxadiazole-2-oxide (intermediate 1)
[0082] Add thiophenol (50ml, 0.50mol) into a reaction flask equipped with mechanical stirring, add 200mL of 5M NaOH aqueous solution under stirring at room temperature, add chloroacetic acid (53g, 0.55mol) after 15min, a large amount of white precipitates are precipitated in the reaction solution; After reaching 140°C, heat and reflux for 2 hours, the reaction solution becomes clear, and turns into a white paste after cooling; slowly add 6N HCl dropwise in an ice bath to adjust the pH to 1, filter, and dry to obtain a crude product, which is washed with H 2 O was recrystallized to obtain 68 g of white solid (97% yield): mp.60-62°C.
[0083] 2) Preparation of 3-benzenesulfonyl-4-hydroxyethyl ether-1,2,5-oxadiazole-2-oxide (formula (Ⅲ)) ...
Embodiment 2
[0094]
[0095] Preparation method of 13-O-(4-oxobutanoic acid-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxo)butyl ester)-β-elemene Basically the same as Example 1, the difference is that the ethylene glycol in step 2) of Example 1 is replaced by Butanediol.
[0096] In this example, a colorless liquid product was obtained with a yield of 85%.
[0097] The 13-O-(4-oxobutanoic acid-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxygen) butyl ester)-β-elemene The structural characterization results are as follows:
[0098] 1 H NMR (300MHz, CDCl 3 )δ8.12–8.01(m,2H),7.77(dd,J=10.7,4.3Hz,1H),7.63(t,J=7.7Hz,2H),5.81(dd,J=17.8,10.5Hz,1H ),5.05(s,1H),5.01(s,1H),4.93(d,J=3.4Hz,1H),4.88(d,J=1.0Hz,1H),4.82(s,1H),4.61(s ,2H),4.58(s,1H),4.46(t,J=6.2Hz,2H),4.20(t,J=6.2Hz,2H),2.76–2.62(m,4H),2.08–1.91(m, 4H),1.90–1.75(m,3H),1.71(s,3H),1.69–1.55(m,3H),1.53–1.41(m,3H),1.00(s,3H).
[0099] 13 C NMR (75MHz, CDCl 3 )δ171.77,171.52,158.42,149.50,147.72,146.88,137.52,135.16,129.20,12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com