Method for preparing sulfenamide thiofide by catalyzing oxidization of molecular oxygen in water phase
A technology of sulfenamides and rubber accelerators, which is applied in the field of catalyzing molecular oxygen oxidation to prepare sulfenamides rubber vulcanization accelerators, can solve the problems of difficult to achieve clean production, a large amount of salt-containing wastewater, production safety problems, etc., and achieves strong performance. Prospects for industrial applications, high reaction efficiency, and the effect of less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Catalytic oxidation of 2-mercaptobenzothiazole (M) in an oxygen atmosphere to synthesize a sulfenamide rubber accelerator.
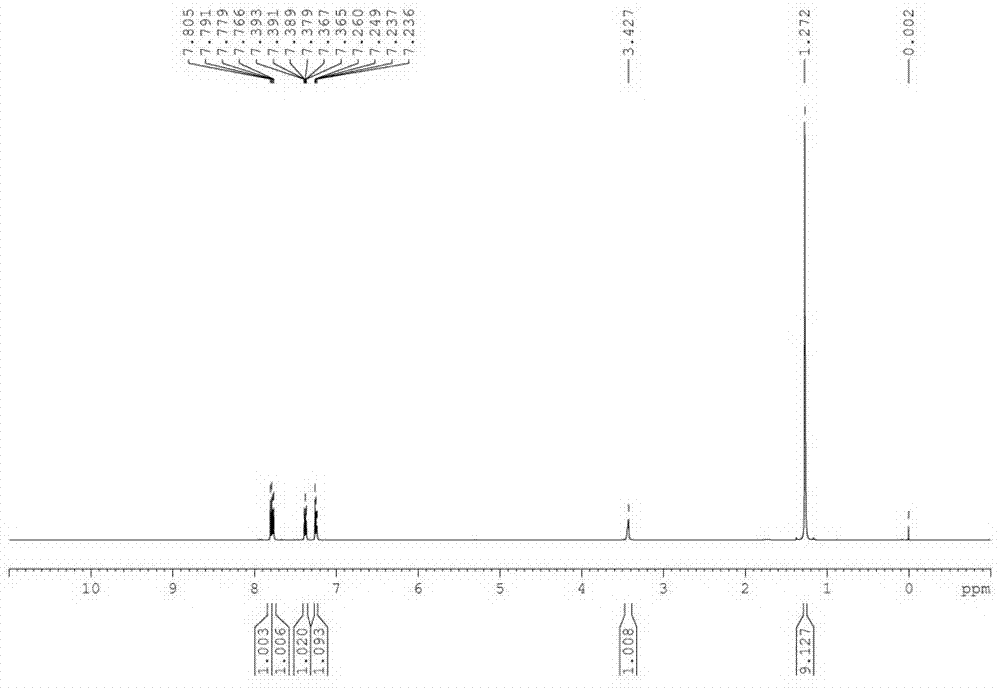
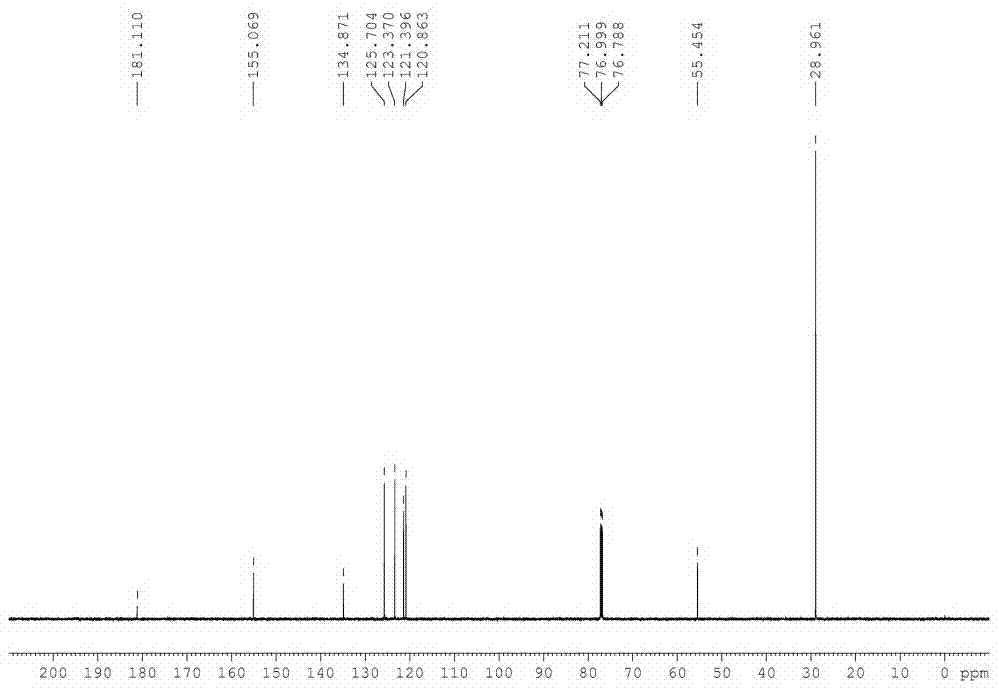
[0035] In a 500 L reactor, put 60 Kg 2-mercaptobenzothiazole, 78.7 Kg tert-butylamine (3 eq), 0.48Kg tetracarboxymanganese tetrasodium phthalocyanine [MnPc(COONa) 4 ] and 180 L of water; heated to 40 °C under stirring, and oxygen was introduced to keep the pressure in the reactor at 0.15 MPa. After 15 hours of reaction, the reaction was stopped, cooled, filtered, washed with 20 L of water, and dried to obtain a white solid 83.8 Kg, the structure of the product was determined to be N-tert-butyl-2-benzothiazolesulfenamide (TBBS) by NMR, MS and other methods, the yield was 98%, and the purity of the product analyzed by liquid chromatography was 99.5%.
[0036] Filter and separate the mother liquor of N-tert-butyl-2-benzothiazole sulfenamide and return to the reactor, re-drop 60 Kg of 2-mercaptobenzothiazole, no need to add catalyst, contin...
Embodiment 2
[0037] Example 2: Synthesis of sulfenamide compounds by catalytic oxidation of 2-mercaptobenzothiazole (M) in air atmosphere.
[0038] In a 3000 L reactor, put 80 Kg 2-mercaptobenzothiazole, 175 Kg tert-butylamine (5 eq), 1.6 Kg cobalt tetrasodium tetracarboxyphthalocyanine [CoPc(COONa) 4 ], 0.8 Kg tetracarboxycopper tetrasodium phthalocyanine [CuPc(COONa) 4 ], and 1600 L of water; heated to 90 ° C under stirring, and air was pressed in to keep the pressure in the reactor at 1.0 MPa. After 18 hours of reaction, the reaction was stopped, cooled, filtered, washed with 100 L of water, and dried to obtain white Solid 110.6 Kg, yield is 97%, and liquid chromatography analysis product purity is 99%.
Embodiment 3
[0039] Example 3: Synthesis of organic matter containing sulfur-nitrogen bonds in an oxygen-enriched air atmosphere.
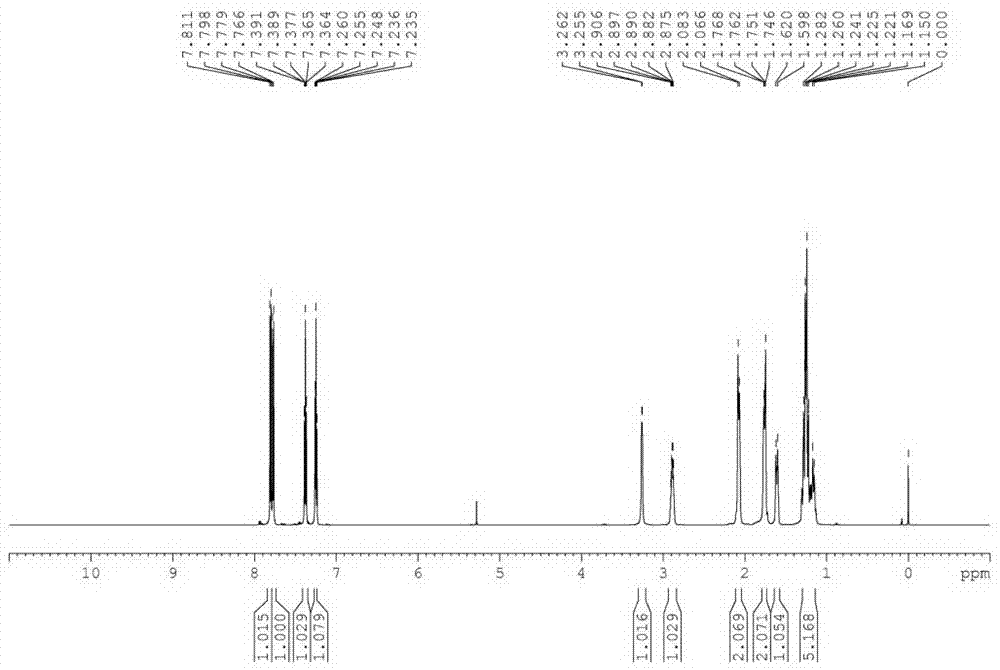
[0040] In a 300 L reactor, put 30 Kg 2-mercaptobenzothiazole, 36 Kg cyclohexylamine (2 eq), 40 g tungsten tetrapotassium tetrasulfonic acid phthalocyanine [WPc(SO 3 K) 4 ], 30 g nickel tetracarboxyphthalocyanine [NiPc(COOH) 4 ], 20 g cesium tetrasulfonate phthalocyanine [CePc(SO 3 h) 4 ] and 90 L of water; heated up to 65°C under stirring, and pressurized oxygen-enriched air [oxygen:air (wt:wt) = 1:2], kept the pressure in the reactor at 0.05 MPa, and stopped the reaction after 8 hours of reaction. Cool, filter, wash with water, and dry to obtain 46.1 Kg of the product N-cyclohexyl-2-benzothiazolesulfenamide (CBS), with a yield of 97%. The purity of the product analyzed by liquid chromatography was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com