Phosphonic acid derivatives and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve the problems of low bioavailability, toxic side effects, virus replication rebound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
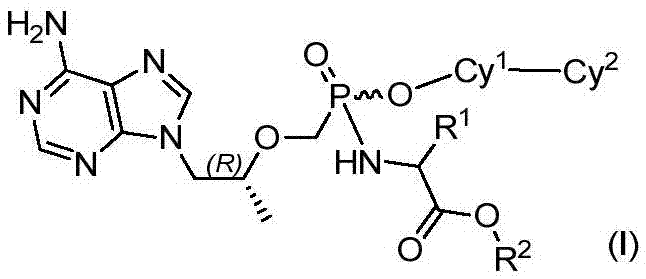
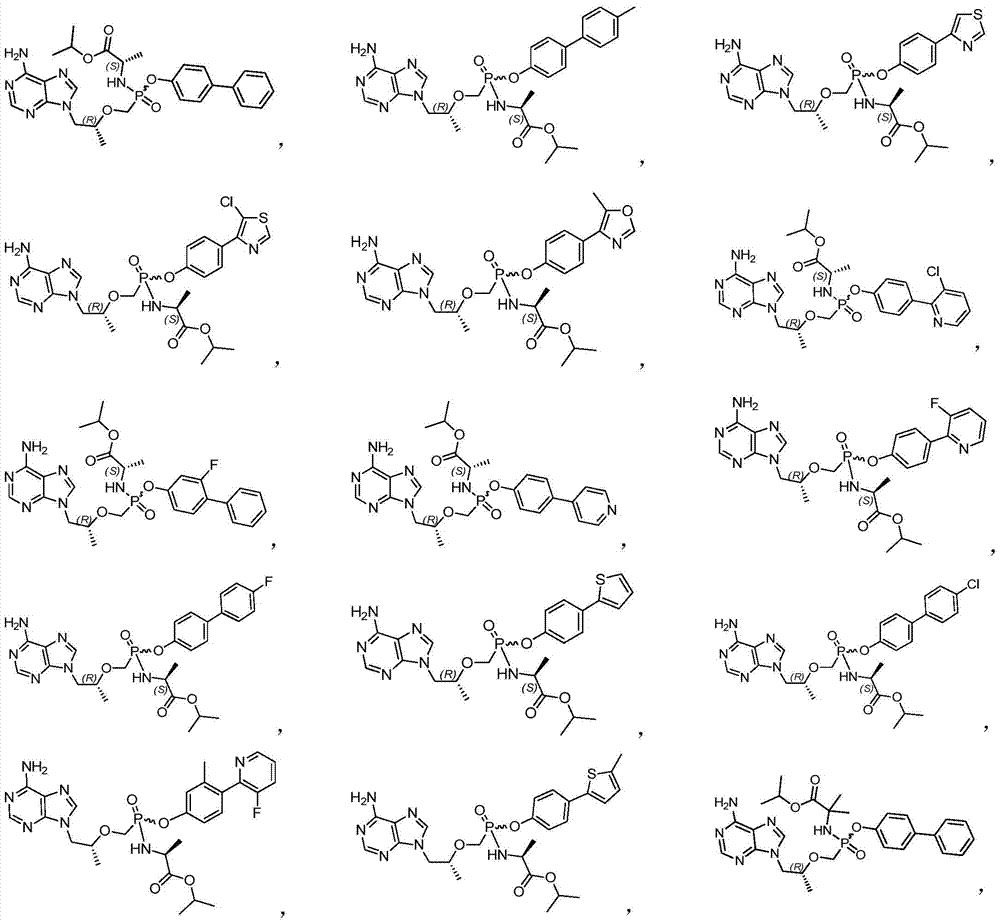
[0081] Example 1 9-[(R)-2-[[[[(S)-1-(isopropoxycarbonyl)ethyl]amino][(biphenyl-4-yl)oxy]phosphinyl] Methoxy]propyl]adenine
[0082]
[0083] Step 1 Preparation of 9-[(R)-2-[[hydroxy[(biphenyl-4-yl)oxy]phosphinyl]methoxy]propyl]adenine
[0084] In a 2L three-necked flask, add p-hydroxybiphenyl (19.0g, 115.0mmol), tenofovir (30.0g, 104.5mmol), 1-(3-dimethylaminopropyl)-3-ethyl Carbodiimide hydrochloride (50 g, 263 mmol), 1,4-dioxane (1300 mL) and pyridine (100 mL). The above reaction system was heated to 100°C and reacted for 12 hours. The reaction solution was distilled off the solvent under reduced pressure to obtain an oily substance, and water (200mL) was added, and the pH value was adjusted to 12 with saturated sodium hydroxide solution to obtain a clear solution, which was extracted with ethyl acetate (3×100mL), and the aqueous phase was stirred. The pH was adjusted to 3 with concentrated hydrochloric acid, and the title compound was precipitated as an off-white soli...
Embodiment 2
[0089] Example 2 9-[(R)-2-[[[[(S)-1-(isopropoxycarbonyl)ethyl]amino][(4'-methylbiphenyl-4-yl)oxy ]phosphinyl]methoxy]propyl]adenine
[0090]
[0091] Step 1 9-[(R)-2-[[[[(S)-1-(isopropoxycarbonyl)ethyl]amino][(4'-methylbiphenyl-4-yl)oxy] Preparation of phosphinyl]methoxy]propyl]adenine
[0092] 9-[(R)-2-[[[[(S)-1-(isopropoxycarbonyl) ethyl]amino][(biphenyl-4 -base) oxy] phosphinyl] methoxy] propyl] adenine, the difference is that the raw material p-hydroxybiphenyl is replaced by 4-(4-methylphenyl) phenol to obtain the title compound . 1 HNMR (500MHz, DMSO-d 6 ):δ8.13(s,2H),7.53-7.63(m,4H),7.10-7.58(m,6H),5.52-5.68(m,1H),4.83-4.87(m,1H),4.30-4.13 (m,2H),3.82-4.10(m,4H),2.32(s,3H),1.02-1.23(m,12H).ESI-MS m / z:567.3[M+H] + .
Embodiment 3
[0093] Example 3 9-[(R)-2-[[[[(S)-1-(isopropoxycarbonyl)ethyl]amino][(4-(thiazol-4-yl)phenoxy]oxy Phosphino]methoxy]propyl]adenine
[0094]
[0095] The preparation of step 1 4-(thiazol-4-yl)phenol
[0096] In a 100mL three-necked flask, add 4-hydroxyphenylboronic acid (10.0g, 61.4mmol), 4-bromothiazole (10.2g, 73.7mmol), potassium carbonate (25.4g, 184.2mmol), 1,4-bis Hexane (30 mmol), water (10 mL) and bistriphenylphosphine palladium dichloride (2.15 g, 3.07 mmol). Under nitrogen protection, the reaction system was heated to 90° C. and stirred for 3 h. The solvent was distilled off by concentration under reduced pressure. The residue was dissolved in ethyl acetate (100 mL), and saturated brine was added for extraction and separation. The combined organic phases were dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product as a light yellow oil, which was purified by column chromatography to obtain the title product as a beige solid. ES...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com