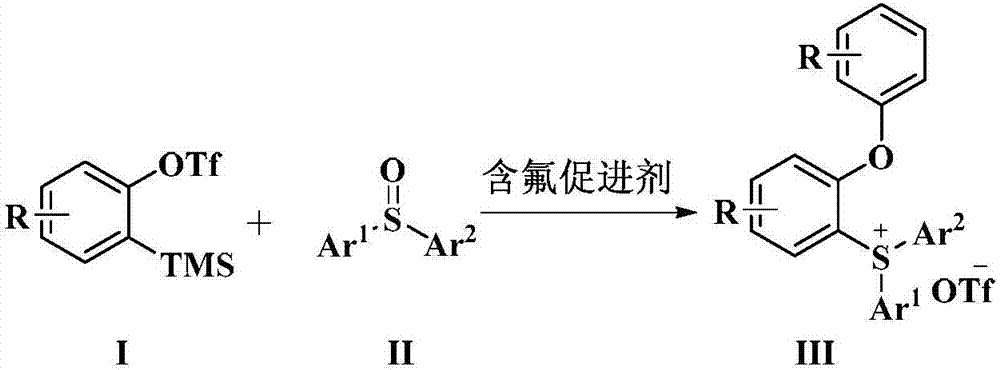
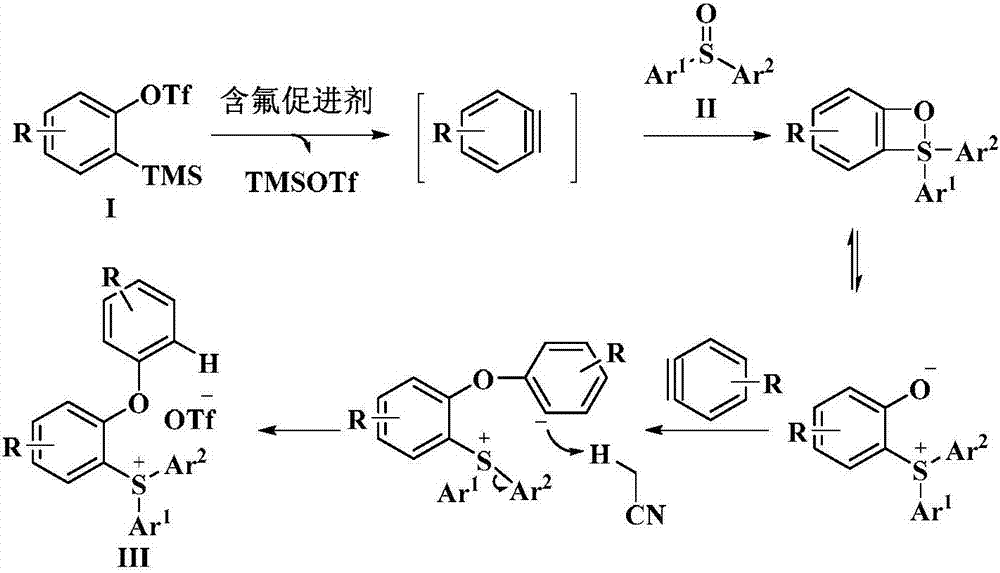
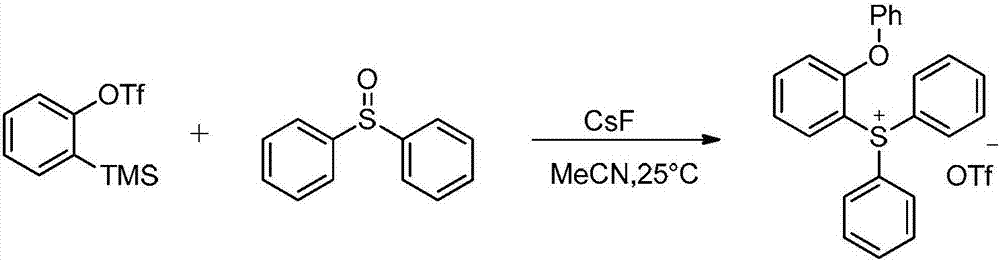
Novel method for preparing triaryl sulfonium salt
A technology of sulfonium salt and triaryl group, which is applied in the field of organic chemical synthesis, can solve the problems of high reaction temperature, strict reaction conditions, and strong alkalinity, and achieve the effects of mild reaction conditions, easy-to-obtain raw materials, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] At room temperature, vacuumize the 25mL reaction tube, change the nitrogen three times, add 91.1mg (0.6mmol) of cesium fluoride (0.6mmol) and acetonitrile (3mL) under the protection of nitrogen, and then add 60.7mg (0.3mmol) of diphenyl sulfoxide, Finally, 89.5 mg (0.3 mmol) of a benzyne precursor was added, and the reaction was carried out at 25°C. Use thin-layer chromatography to track the reaction process. The developing agent is dichloromethane / methanol with a volume ratio of 15:1. The reaction time is 16 hours. Concentrated and separated by column chromatography, the target product was obtained in a yield of 78%.
[0027] In deuterated chloroform at 25°C, the synthetic target compound was tested for 1 H NMR spectrum, where the peaks are assigned as: δ7.83-7.70(m, 10H), 7.66-7.61(m, 1H), 7.36-7.31(m, 3H), 7.23-7.16(m, 2H), 6.95- 6.91(m,1H),6.88-6.82(m,2H). 13 C NMR (151MHz, CDCl 3 ): δ156.8, 153.3, 136.6, 134.9, 131.8, 131.1, 130.4, 126.2, 125.1, 122...
Embodiment 2
[0029] The only difference from Example 1 is that the reaction solvent is dichloromethane, and the yield is 16%.
Embodiment 3
[0031] The only difference from Example 1 is that the reaction solvent is tetrahydrofuran, and the yield is 14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com