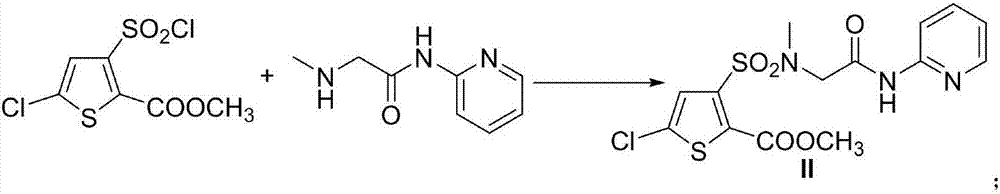
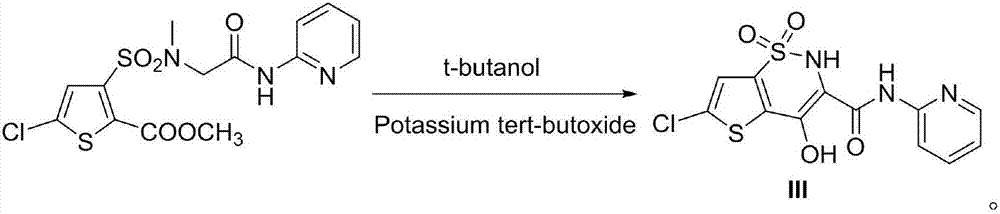
Preparation method of non-steroidal anti-inflammatory analgesic lornoxicam
A technology of non-steroidal anti-inflammatory and lornoxicam, which is applied in the field of medicine, can solve the problems of high toxicity and low yield, and achieve the effect of short process route, high purity and novel route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
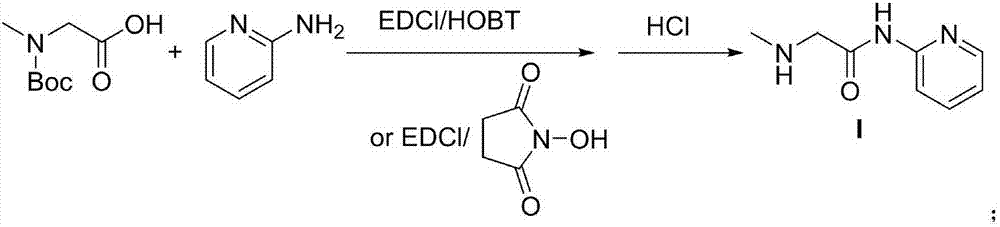
[0022] The first step: the preparation of 2-N-methyl-2-N-Boc-acetyl (2-pyridyl) amine (I)
[0023] Boc-sarcosine 190kg, dissolved in 800kgTHF, add HOBt 150kg, triethylamine 120kg, add 220kg EDCi in batches at zero degree, after adding, react at room temperature for 2h, after the reaction, add 2-amino The tetrahydrofuran solution of pyridine (110Kg 2-aminopyridine dissolved in 150kgTHF) was added dropwise in 1 hour, and reacted overnight at room temperature. Take out after the reaction section, filter, recover THF from the filtrate under reduced pressure, add 300kg of methanol and 500kg of dilute hydrochloric acid to the residue, heat and reflux for 1 hour, the reaction is complete, recover most of the methanol under reduced pressure, add 1mol / L sodium hydroxide solution dropwise at zero temperature, When the pH was about 9, 500kg of ethyl acetate was extracted, dried over anhydrous magnesium sulfate, filtered, and 200kg of ethyl acetate was recovered from the filtrate under re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com