Anti-VEGFR (vascular endothelial growth factor receptor) 2 monoclonal antibody and application thereof
A monoclonal antibody and amino acid technology, applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-tumor drugs, etc., can solve problems that hinder the development of antibody drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Positive antibody (PCAb) gene synthesis, expression vector construction and preparation of antibody
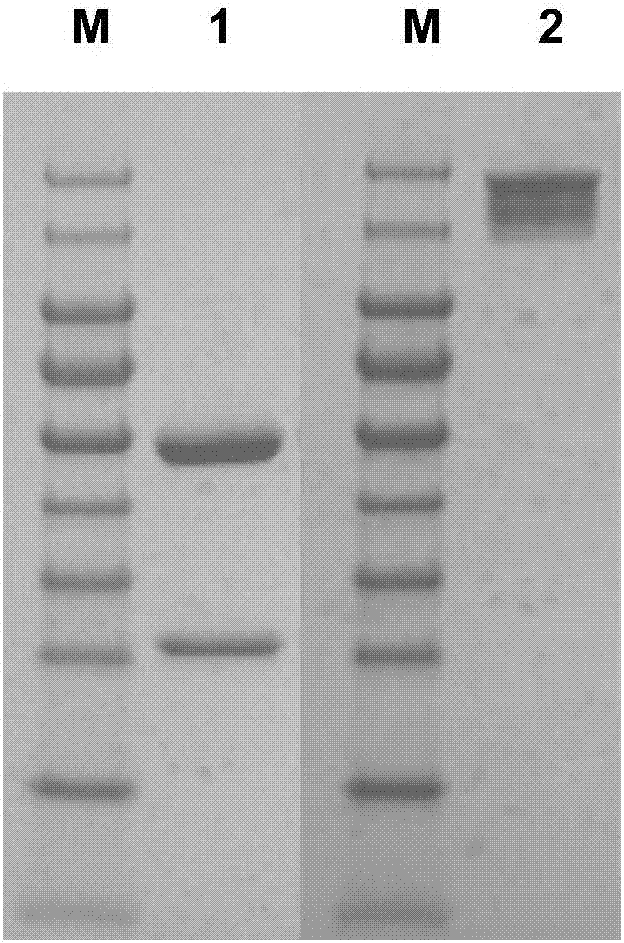
[0063] 1. Referring to the patent CN 1345334A, select an antibody with good biological activity as the positive antibody (PCAb). The amino acid sequence of PCAb is as follows:
[0064] PCAbH, as shown in SEQ ID NO: 13:
[0065] QVKLQQSGAELVGSGASVKLSCTTSGFNIKDFYMHWVKQRPEQGLEWIGWIDPENGDSDYAPKFQGKATMTADSSSNTAYLQLSSLTSEDTAVYYCNAYYGDYEGYWGQGTTVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
[0066] PCAbL, as shown in SEQ ID NO: 14:
[0067] DIELTQSPAIMSASSPGEKVTITCSASSSVSYMHWFQQKPGTSPKLWIYSTSNLASGVPARFSGSGSGTSYSLTISRMEAEDAATYYCQQRSSYPFTFGSGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPR...
Embodiment 2
[0071] Example 2: Construction of natural human single chain antibody phage display library
[0072] 1. Construction of phagemid vector
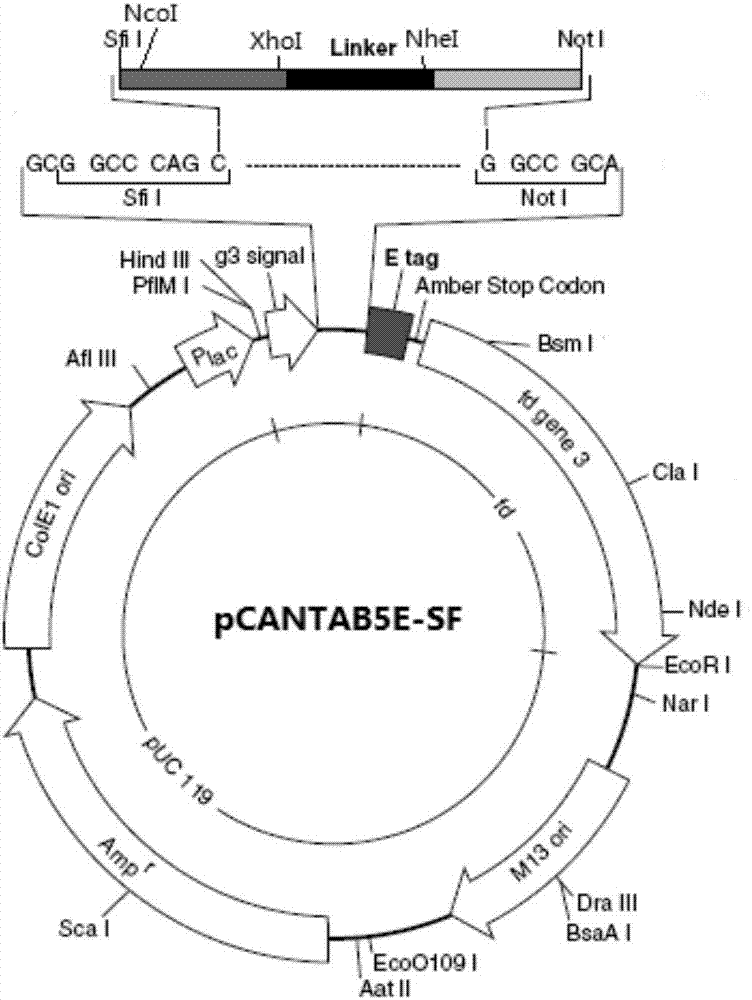
[0073] Select pCANTAB5E as the phage display vector, and carry out vector transformation according to the needs of cloning and phage display. The transformation results are as follows: figure 2. The SfiI-NcoI-XhoI+Linker+NheI-NotI sequence (SED ID NO: 15) was gene-synthesized, then digested with SfiI and NotI, and ligated with the pCANTAB5E vector to obtain the transformed vector pCANTAB5E-SF.
[0074] 2. PBMC isolation and mRNA extraction
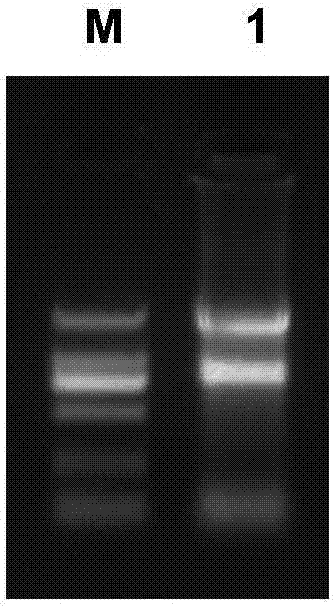
[0075] Aseptically extract fresh peripheral blood from healthy volunteers, use lymphocyte separation medium (GE) to separate the lymphocytes therein, and use Invitrogen’s reagent (15596-026) extracts 100×10 6 The total RNA of each cell, the result is as follows image 3 shown.
[0076] 3. Antibody library primer design, synthesis and RT-PCR
[0077] According to the antibody gene sequence inform...
Embodiment 3
[0090] Example 3: Phage display and screening of human antibody library
[0091] 1. Phage display and panning of antibody library
[0092] Inoculate 880ml of 2YT-AG medium (containing 100μg / ml ampicillin and 2% glucose) with 100 times the library capacity of the above-mentioned human VH and VL single-chain antibody library, culture at 37°C and 200rpm until OD600=0.5-0.6, add Helper phage with a cell density of 100 times, infect for 1.5 hours, collect the bacteria by centrifugation, resuspend the cells in 400ml 2YT-AK medium (containing 100μg / ml ampicillin and 75μg / ml kanamycin), and culture overnight at 30°C and 200rpm .
[0093] Centrifuge the culture from the previous step at 10,000g at 4°C for 20min, collect the supernatant and add 1 / 4 volume of PEG / NaCl, mix well, and let it stand on ice for 1h; centrifuge at 12,000g at 4°C for 25min, discard the supernatant, and place the centrifuge tube upside down on the plate Remove the liquid on the paper; resuspend the phage pellet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com