1,2,3,4,6-O-pentagalloylglucose molecular imprinting monolithic column preparation method
A pentagalloylglucose, molecular imprinting technology, applied in separation methods, chemical instruments and methods, solid adsorbent liquid separation, etc., can solve problems such as unachievable purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
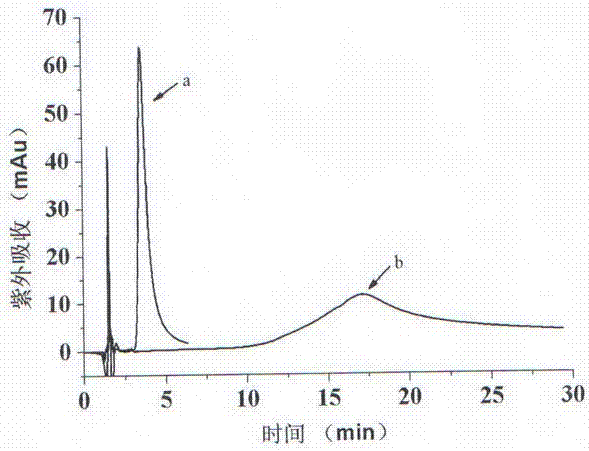
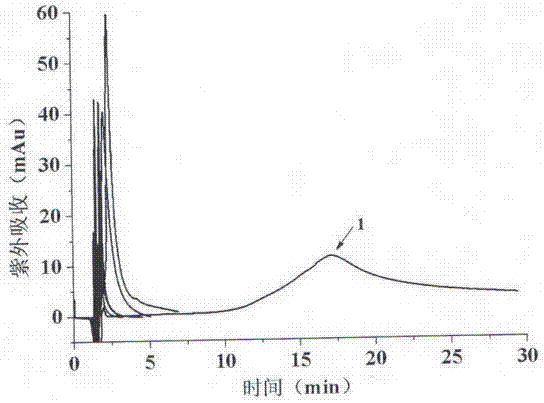
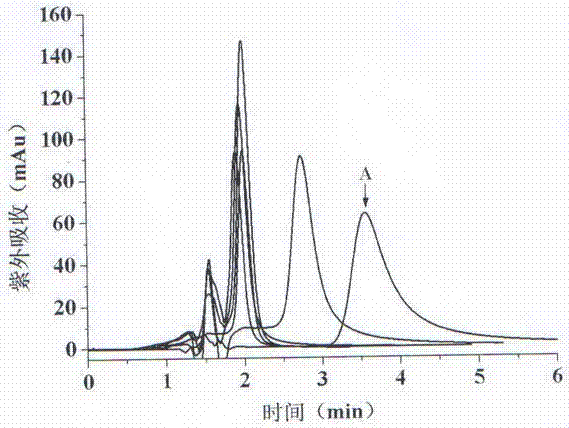
Image
Examples
Embodiment 1
[0020] Preparation of 1,2,3,4,6-O-pentagalloyl glucose molecularly imprinted monolithic column by in situ polymerization:
[0021] a. Dissolve 47.04 mg of 1,2,3,4,6-O-pentagalloyl glucose and 32 μL of 4-vinylpyridine in 240 μL of ternary porogen N,N-dimethylformamide, dimethyl sulfoxide 1200 μL and 2468 μL of 1-butyl-3-methyltetrafluoroborate, then add 8.84 mg of nickel acetate and 20 mg of free radical initiator azobisisobutyronitrile, then add 630 μL of ethylene glycol dimethacrylate and 171.5 μL of methyl ether methacrylate oligomer with an average relative molecular mass of 300 g / mol, ultrasonicated for 30 minutes to completely dissolve and mix all components to obtain a homogeneous solution, then fill the solution with nitrogen gas to remove the dissolved The oxygen in it is transferred to the stainless steel tube, and the two ends of the stainless steel tube are sealed, and the stainless steel column is placed in a constant temperature water bath with a temperature of 60...
Embodiment 2
[0025] Preparation of 1,2,3,4,6-O-pentagalloyl glucose molecularly imprinted monolithic column by in situ polymerization:
[0026] a. Dissolve 28.22 mg of 1,2,3,4,6-O-pentagalloyl glucose and 32 μL of 4-vinylpyridine in 240 μL of ternary porogen N,N-dimethylformamide, dimethyl sulfoxide 1200 μL and 2468 μL of 1-butyl-3-methyltetrafluoroborate, then add 8.84 mg of nickel acetate and 20 mg of free radical initiator azobisisobutyronitrile, then add 630 μL of ethylene glycol dimethacrylate and The average relative molecular weight is 300g / mol methyl ether methacrylate oligomer 171.5μL, sonication for 30 minutes, so that all components are completely dissolved and mixed to obtain a homogeneous solution, and then the solution is filled with nitrogen to remove the dissolved oxygen, transfer the solution to a stainless steel tube, and seal both ends of the stainless steel tube, and put the stainless steel column into a constant temperature water bath at a temperature of 60°C for 18 ho...
Embodiment 3
[0030] Preparation of 1,2,3,4,6-O-pentagalloyl glucose molecularly imprinted monolithic column by in situ polymerization:
[0031] a. Dissolve 14.12 mg of 1,2,3,4,6-O-pentagalloyl glucose and 32 μL of 4-vinylpyridine in 240 μL of ternary porogen N,N-dimethylformamide, dimethyl sulfoxide 1200 μL and 2468 μL of 1-butyl-3-methyltetrafluoroborate, then add 8.84 mg of nickel acetate and 20 mg of free radical initiator azobisisobutyronitrile, then add 630 μL of ethylene glycol dimethacrylate and 171.5 μL of methyl ether methacrylate oligomer with an average relative molecular mass of 300 g / mol, ultrasonicated for 30 minutes to completely dissolve and mix all components to obtain a homogeneous solution, and then fill the solution with nitrogen gas to remove the dissolved Oxygen in it, transfer the solution to a stainless steel tube, and seal both ends of the stainless steel tube, put the stainless steel column in a constant temperature water bath with a temperature of 60°C for 18 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com