Mesenchymal stem cell serum-free culture medium
A technology of serum-free medium and basal medium, applied in the direction of cell culture active agent, culture process, tissue culture, etc., can solve the problem of not supporting primary cell culture, complex components, and inability to effectively maintain the differentiation potential of mesenchymal stem cells and other problems, to achieve the effect of clear ingredients and simple addition of ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation of mesenchymal stem cell serum-free medium
[0049] This example provides a serum-free medium for mesenchymal stem cells, using DMEM / F12 as the basal medium, and adding the following components to the basal medium:
[0050]
[0051]
[0052] See the table below for the cell culture reagents and cytokine manufacturers and article numbers used in this example:
[0053]
[0054]
[0055] Adjust the parameters of the medium as follows:
[0056] pH: 7.2-7.4
[0057] Osmotic pressure: 260-320mosm
[0058] Bacteria, fungi test: negative
[0059] Chlamydia, mycoplasma detection: negative
[0060] Endotoxin: 0-0.5EU / mL
[0061] The complete medium prepared above was sterilized by filtration with a 0.22um filter, and stored at -20°C in the dark.
Embodiment 2
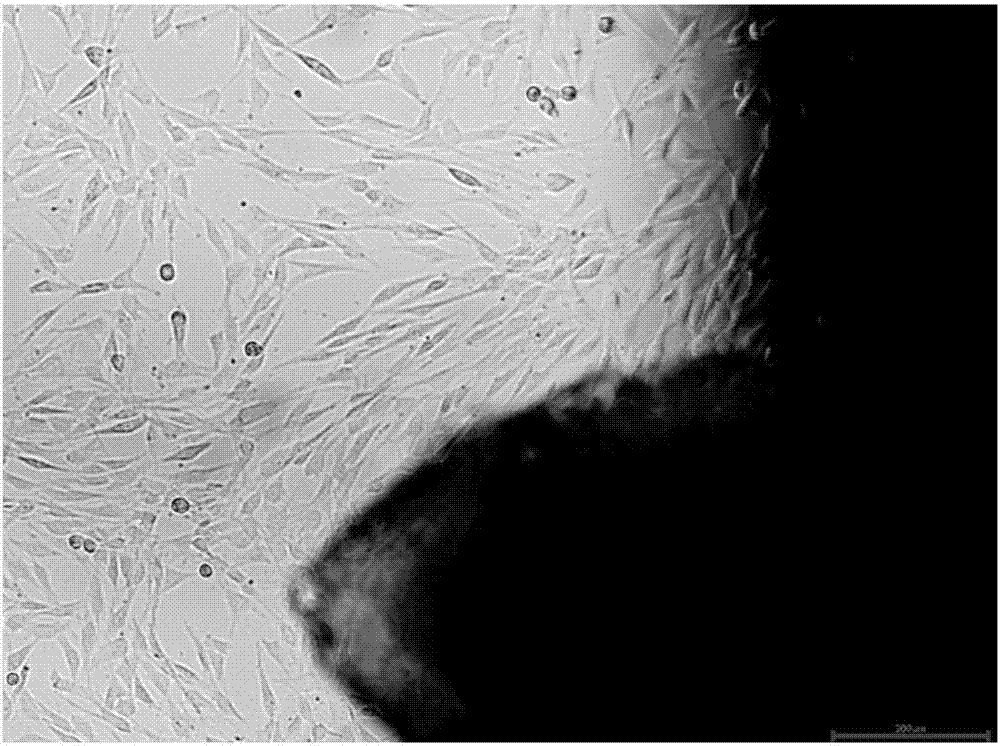
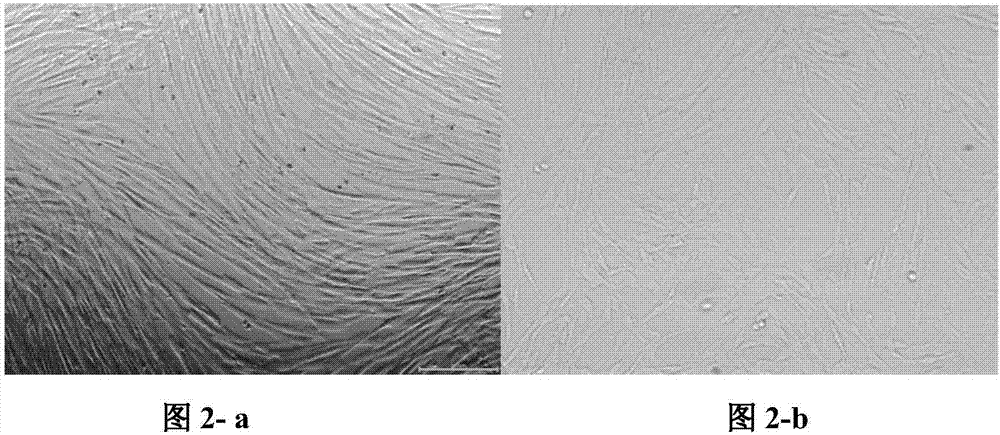
[0062] Embodiment 2: preparation of human umbilical cord mesenchymal stem cells
[0063] Separate primary cultures with the following three groups of media:
[0064] (1) Group 1: the serum-free medium for mesenchymal stem cells according to Example 1 of the present invention;
[0065] (2) Group 2: traditional serum-containing medium (DMEM / F12+10% FBS);
[0066] (3) Group 3: serum-free medium for human mesenchymal stem cells (produced by LONZA, product number 00190632).
[0067] Experimental steps:
[0068] (1) Under aseptic conditions, the umbilical cords of healthy newborns (including the blood test results of the puerpera's physical examination) were collected, both ends were ligated with silk thread, and soaked in a preservation bottle containing 2% double antibody (penicillin and streptomycin mixture).
[0069] (2) Take out the umbilical cord from the biological safety cabinet, disinfect the surface of the umbilical cord with 75% medical alcohol for 30-40 seconds, cut o...
Embodiment 3
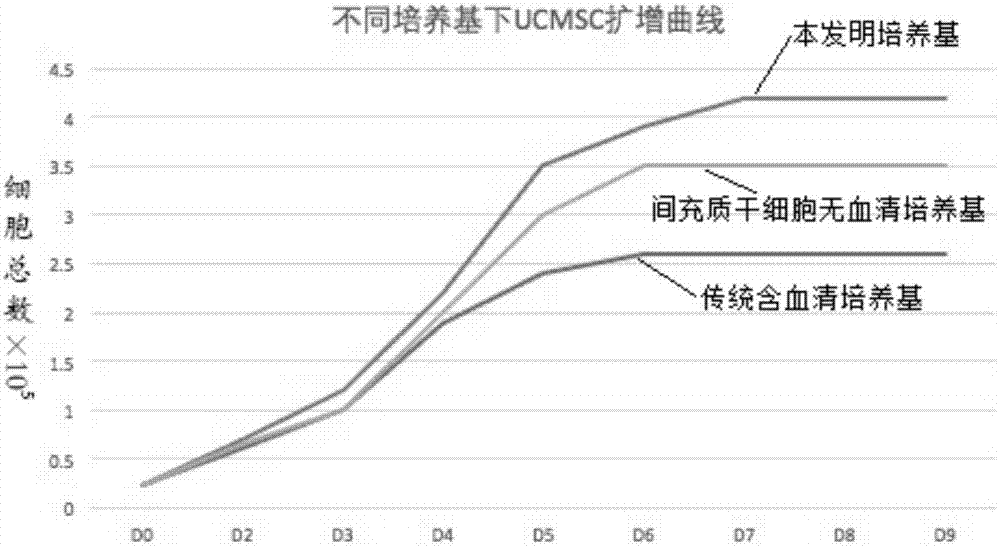
[0074] Embodiment 3: Three groups of cultured umbilical cord mesenchymal stem cell proliferation ability contrast
[0075] Experimental procedure: take the three groups of medium in Example 2 to culture well-growing P3 human umbilical cord mesenchymal stem cells, enzymatically hydrolyze to make a single cell suspension, and adjust the concentration to 2.4x 10 4 cells / mL, add to 12-well plate, add corresponding three groups of media respectively, 1 mL per well, place at 37°C, 5% CO 2 Culture in an incubator. From the second day, take three wells of each group every other day to calculate the cell volume, calculate the average value, and measure continuously for 8 days. Take the culture time as the horizontal axis and the total number of cell proliferation as the vertical axis to draw the cell growth curve . Such as image 3 shown.
[0076] from image 3 It can be seen that when using the medium of Example 1 of the present invention to culture mesenchymal stem cells, the cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com