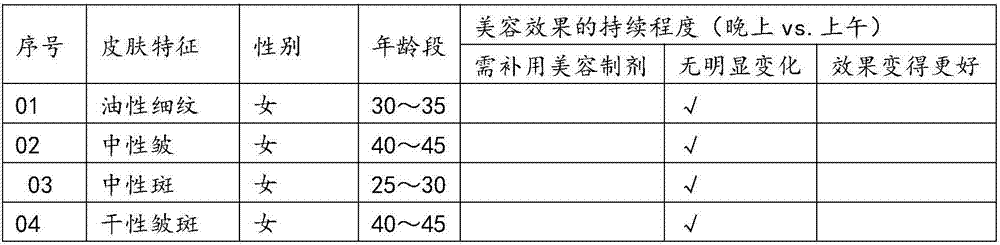
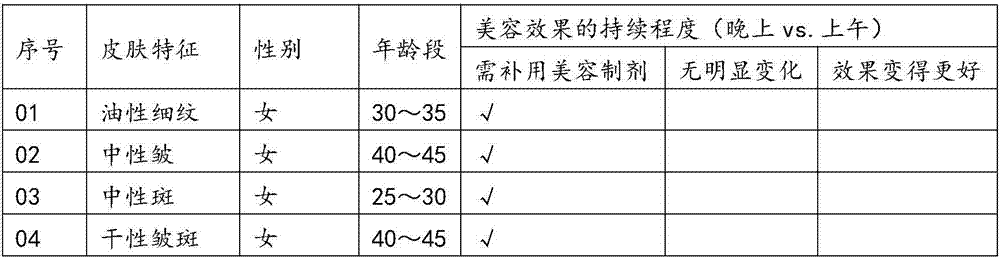
Preparing method and application of human mesenchymal stem cell source exosome bio-active preparation
A technology of stem cells and exosomes, applied in the field of biomedicine, can solve the problem of insignificant anti-aging effect, achieve large market prospects and economic value, and save time and cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 This example discloses a preparation method of cosmetic preparations, the specific steps are as follows:
[0038] 1) Weigh 200 mg of chitosan and dissolve it in 100 mL of 0.5% dilute acetic acid to obtain a chitosan solution.
[0039] 2) Weigh 5 mg of human mesenchymal stem cell-derived exosome freeze-dried powder, 10 mg of glucosaminoglycan, and 10 mg of hyaluronic acid, and dissolve them in 50 mL of glycerol to obtain an exosome solution.
[0040] 3) Slowly add the exosome solution into the chitosan solution dropwise under continuous stirring, and react for 30 minutes to obtain the chitosan nanoparticle suspension of the exosome.
[0041] 4) The suspension was centrifuged at 4 times at high speed (10000r / min) for 30 minutes, the precipitate was collected, washed 3 times with water for injection, and dried to obtain the human mesenchymal stem cell-derived exosome sustained-release cosmetic preparation.
Embodiment 2
[0042] Example 2 This example discloses the method for preparing the freeze-dried powder of exosomes described in Example 1
[0043] Take exosomes, add mannitol (1%-15%), mix well, filter (0.22 μm), sub-package, and then place in an ultra-low temperature refrigerator (-80°C) for 10h. Then, freeze-dry (-50° C., 24 h) under a vacuum of 10 Pa to obtain a freeze-dried powder of human mesenchymal stem cell-derived exosomes.
Embodiment 3
[0044] Example 3 This example discloses the preparation method of exosomes described in Example 2
[0045] A. Human MSC culture and pretreatment process:
[0046] 1. Materials and reagents
[0047] 1) For MSCs from human umbilical cord, placenta, bone marrow, fat or other sources, the number of passages can be within 6 generations.
[0048] 2) Human MSC serum-free medium
[0049] 3) alpha-MEM
[0050] 4) Recombinant human interferon
[0051] 5) Recombinant human EPO
[0052] 6) Recombinant human PDGF-BB
[0053] 7) Petri dish (diameter 150mm)
[0054] 8) Other consumables
[0055] B. Cell culture process
[0056] 1) Digest and count human subcultured MSCs, suspend them in MSC serum-free complete medium, and adjust the cell concentration to 1.0×10 6 / ml.
[0057] 2) according to 10 6 Inoculate in a 150mm large Petri dish and culture until the cell confluency reaches 70-80%. It usually takes 2-3 days.
[0058] 3) Aspirate the liquid in the petri dish, and wash the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com