Amphipathic polypeptide molecule capable of being used as gene vector
A gene carrier and amphipathic technology, applied in the field of genetic engineering, can solve the problems of non-degradability and strong cytotoxicity, and achieve the effects of efficient gene transfection, high transfection efficiency and high transfection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of amphiphilic polypeptide molecules that can be used as gene carriers (take the synthesis of 0.25mmol peptide molecules as an example)
[0031] 1. Materials
[0032] (1) Weigh 0.982g of MBHA resin with a loading capacity of 0.318mmol / g, and swell in DCM overnight;
[0033] (2) Prepare a DMF (dimethylformamide) solution of the following amino acids at a concentration of 0.2mol / L:
[0034] Fmoc-Ala-OH (N-fluorenylmethoxycarbonyl-alanine): volume 11mL, mass 0.82g;
[0035] Fmoc-Gly-OH (N-fluorenylmethoxycarbonyl-glycine): volume 56mL, mass 1.90g;
[0036] Fmoc-Pro-OH (N-fluorenylmethoxycarbonyl-proline): volume 11mL, mass 0.74g;
[0037] Fmoc-Ile-OH (N-fluorenylmethoxycarbonyl-isoleucine): volume 32mL, mass 2.26g;
[0038] Fmoc-Lys(Boc)-OH(N-fluorenylmethoxycarbonyl-N'-tert-butoxycarbonyl-lysine): volume 32mL, mass 3.00g;
[0039] Fmoc-Arg(Pbf)-OH(N-fluorenylmethoxycarbonyl-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-arginine): volume 84mL, mass 10.9...
Embodiment 2
[0052] Morphology and secondary structure of amphiphilic polypeptide molecules that can be used as gene carriers
[0053] Prepare a series of Tris buffer (pH 7.0) for amphiphilic polypeptide molecules, the molecular sequence is as follows:
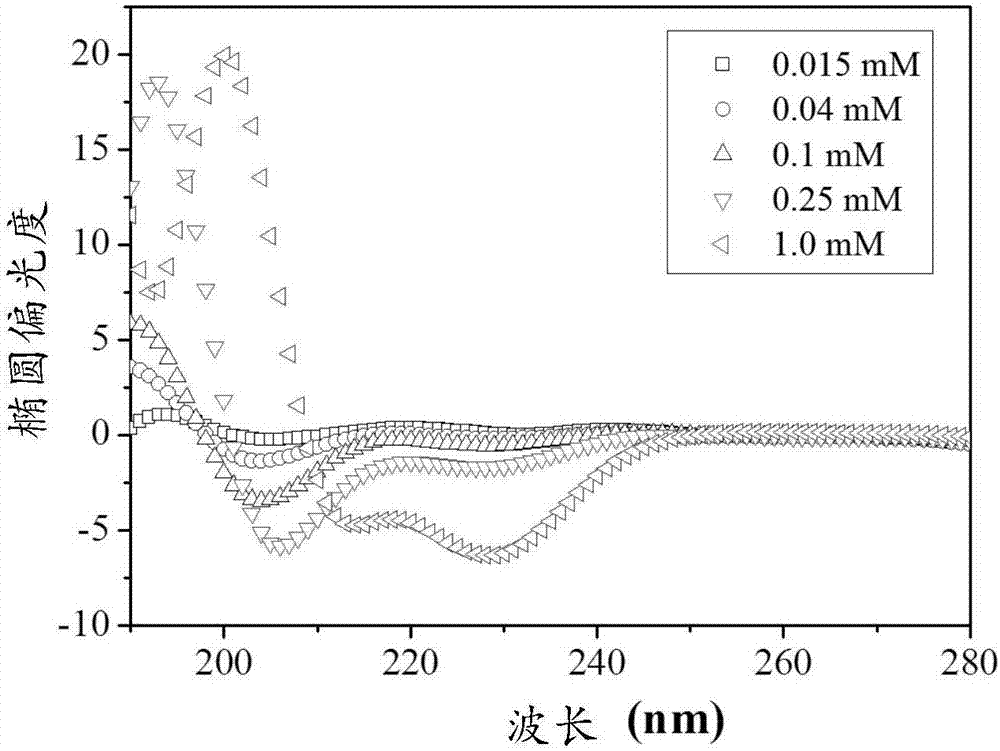
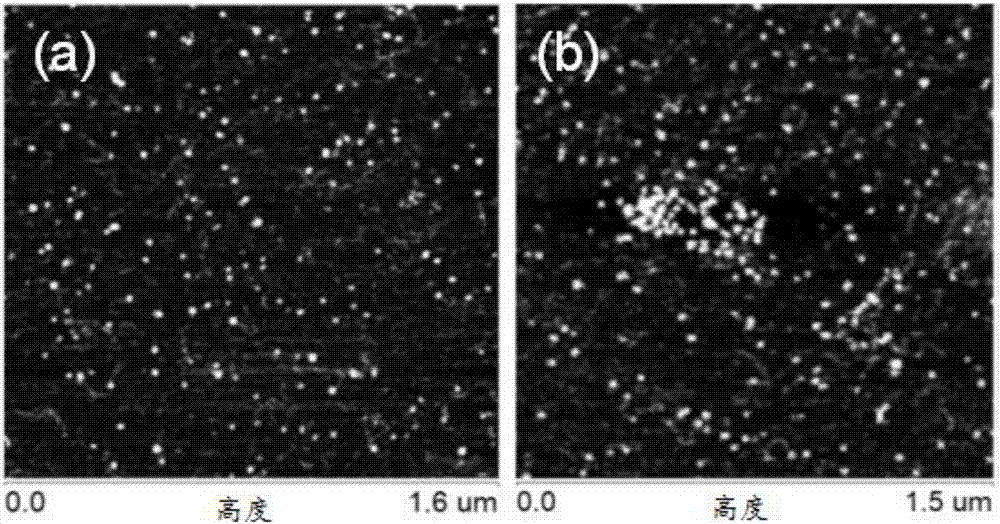
[0054] Ac-Arg-Gly-Asp-Gly-Pro-Leu-Gly-Leu-Ala-Gly-Ile-Ile-Ile-Gly-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-NH 2 , the concentration of which is 0.015mM, 0.040mM, 0.100mM, 0.250mM and 1.0mM, respectively, at room temperature for 3 days, observe the results of circular dichroism spectrum and atomic force microscope.
[0055] The results of the circular dichroism spectrum are as follows figure 1 As shown, it is found that the molecule is in the α-helical secondary structure within the concentration range of the prepared solution; the results of atomic force microscopy are as follows figure 2 As shown, it was found that many spherical aggregates existed in the solution below the concentration of 1.0mM, with a diameter of 5-100nm.
Embodiment 3
[0057] Morphology and structure exploration of amphiphilic polypeptide molecules that can be used as gene carriers after mixing with λ-DNA molecules
[0058] The amphiphilic polypeptide molecule, the molecular sequence is as follows:
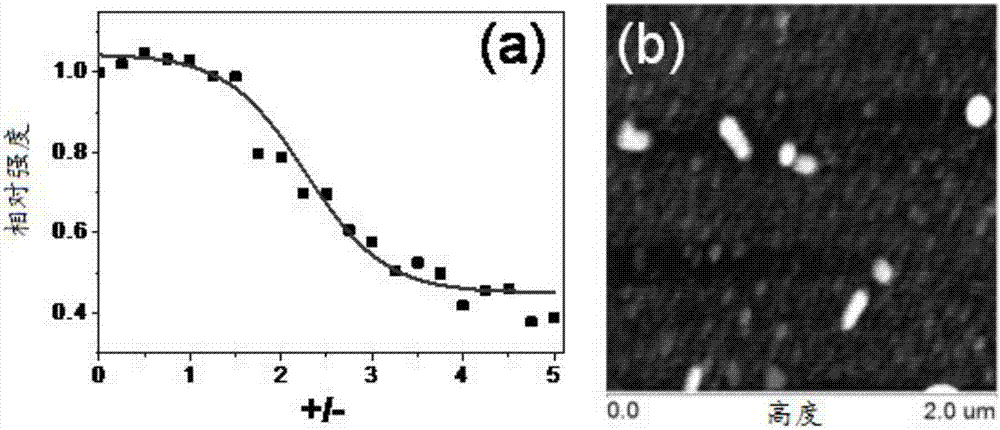
[0059] Ac-Arg-Gly-Asp-Gly-Pro-Leu-Gly-Leu-Ala-Gly-Ile-Ile-Ile-Gly-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-NH 2 Mix with λ-DNA molecules, wherein the concentration of immobilized λ-DNA is 10 μg / mL, and the concentration of 1.54×10 3- mM ethidium bromide EtBr, control the concentration of amphiphilic polypeptide molecules, so that the ratio (+ / -) of the number of positive charges to the number of negative charges of λ-DNA molecules increases from small to large, and observe the mixed system at a wavelength of 600nm Fluorescence intensity changes (excitation wavelength: 520nm).
[0060] Fluorescence intensity changes as a result of image 3 As shown in a, the fluorescence intensity decreases with the increase of the ratio (+ / -), and reaches an equilibri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com