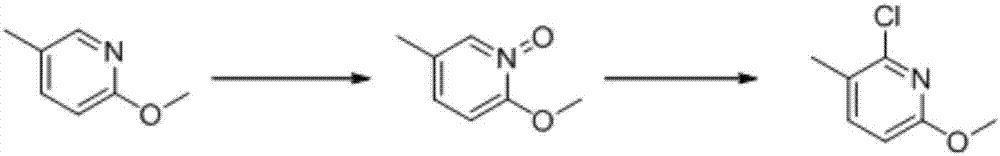
Preparation method of 2-chloro-6-methoxy-3-methylpyridine
A kind of methylpyridine and methoxyl technology, applied in the field of organic chemical synthesis, can solve the problem that there is no literature report 2-chloro-6-methoxy-3-methylpyridine preparation method, chlorination temperature and chlorinating agent sensitivity , the problem of high production and operation cost, to achieve the effect of low cost, good stability and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A preparation method of 2-chloro-6-methoxy-3-methylpyridine, said preparation method comprising the steps of:
[0025] (1) Add 2-methoxy-5-methylpyridine (50g, 0.4mol) and 40% hydrogen peroxide (100mL) into the solvent acetic acid (300mL) and mix them, and react for 1h at 80°C. The reaction solution was poured into crushed ice, and kept stirring, and then extracted with dichloromethane. After the organic phase was concentrated, 55 grams of a white solid product was obtained, that is, the 2-methoxy-5-methylpyridine-N-oxide (Yield 97%, content 96%).
[0026] (2) Add 2-methoxy-5-methylpyridine-N-oxide (50g, 0.36mol) and phosphorus oxychloride (200mL) into carbon tetrachloride (250mL) and mix them at 50°C 5h, after the reaction was completed, the solvent was removed by rotary evaporation, and then the reaction product was poured into crushed ice and kept stirring, then extracted with dichloromethane, and the organic phase was washed, dried, and concentrated to obtain 48g o...
Embodiment 2
[0029] A preparation method of 2-chloro-6-methoxy-3-methylpyridine, said preparation method comprising the steps of:
[0030] (1) Add 2-methoxy-5-picoline (50g, 0.4mol) and carbamide peroxide (330g, 3.5mol) into the solvent trifluoroacetic anhydride (250mL) and mix them, and react at 120°C for 15h, After the reaction was completed, the reaction solution was poured into crushed ice, and kept stirring, then extracted with dichloromethane, and after the organic phase was concentrated, 56 grams of a white solid product was obtained, that is, the 2-methoxy-5-picoline- N-oxide (yield 99%, content 97%).
[0031] (2) Add 2-methoxy-5-methylpyridine-N-oxide (50g, 0.36mol) and thionyl chloride (300mL) into dichloroethane (200mL) and mix, and react at 80°C 18h, after the reaction was completed, the solvent was removed by rotary evaporation, and then the reaction product was poured into crushed ice and stirred continuously, then extracted with dichloromethane, and the organic phase was wa...
Embodiment 3
[0033] A preparation method of 2-chloro-6-methoxy-3-methylpyridine, said preparation method comprising the steps of:
[0034] (1) Add 2-methoxy-5-methylpyridine (50g, 0.4mol) and m-chloroperoxybenzoic acid (1.3kg, 8.0mol) into the solvent trifluoroacetic acid (500mL) and mix them at 150°C After the reaction was completed, the reaction solution was poured into crushed ice and kept stirring, then extracted with dichloromethane, and after the organic phase was concentrated, 52 grams of a white solid product was obtained, namely the 2-methoxy-5-methanol Pyridine-N-oxide (yield 92%, content 97%).
[0035] (2) Add 2-methoxy-5-methylpyridine-N-oxide (50g, 0.36mol) and phosphorus oxychloride (750g, 3.6mol) into chloroform (250mL) and mix, and react at 130°C 5h, after the reaction was completed, the solvent was removed by rotary evaporation, and then the reaction product was poured into crushed ice and stirred continuously, then extracted with dichloromethane, and the organic phase wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com