Synthetic method of dabigatran etexilate key intermediate
A dabigatran etexilate and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of compound 8 being expensive, not meeting the needs of industrial production, and not easy to obtain, and achieve good industrial production prospects, stable properties, and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
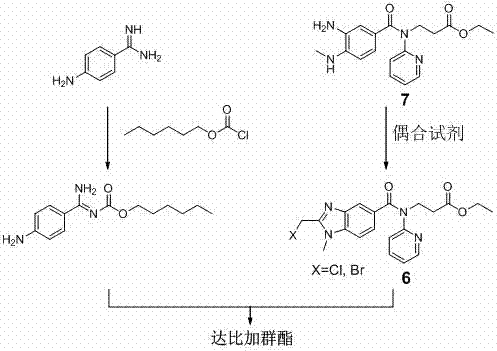
[0035] The synthetic method of dabigatran etexilate key intermediate (formula 6 compound), comprises the following steps:
[0036] 1. Preparation of formula 2 compound
[0037]
[0038] 30.2 g (0.2 mol) of p-methylaminobenzoic acid was mixed with 280 ml of dichloromethane at room temperature and stirred for 1 hour, then 32.2 mL (0.4 mol) of pyridine was added and cooled to -20 o C, slowly add bromine 10.76 mL (0.21 mol), keep the low temperature and continue the reaction for 5 hours, finish the reaction with 50 mL saturated sodium thiosulfate aqueous solution, add 100 mL ethyl acetate, separate the organic phase, and then use 300 mL saturated chlorine Wash with sodium chloride aqueous solution, dry over anhydrous sodium sulfate, concentrate under reduced pressure to about 100 mL, add 50 mL of n-hexane, a solid precipitates, stir at room temperature for 2 hours, filter with suction, wash with 50 mL of dichloromethane, 50 o C was dried in vacuum for 1 hour to obtain 43.0 g o...
Embodiment 2
[0046] 1. Preparation of formula 2 compound
[0047]
[0048] 30.2 g (0.2 mol) of p-methylaminobenzoic acid was mixed with 280 ml of tetrahydrofuran at room temperature and stirred for 1 hour, then 55.7 mL (0.4 mol) of triethylamine was added and cooled to -20 o C, slowly add bromine 10.76 mL (0.21 mol), keep the low temperature and continue the reaction for 5 hours, finish the reaction with 50 mL saturated sodium thiosulfate aqueous solution, add 100 mL ethyl acetate, separate the organic phase, and then use 300 mL saturated chlorine Wash with sodium chloride aqueous solution, dry over anhydrous sodium sulfate, concentrate under reduced pressure to about 100 mL, add 50 mL of n-hexane, a solid precipitates, stir at room temperature for 2 hours, filter with suction, wash with 50 mL of dichloromethane, 50 o C was dried in vacuum for 1 hour to obtain 43.0 g of white powdery solid, yield: 94%.
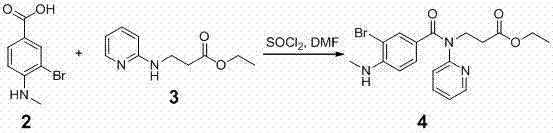
[0049] 2. Preparation of the compound of formula 4
[0050]
[0051] Mix 34.4 ...
Embodiment 3
[0056] 1. Preparation of formula 2 compound
[0057]
[0058] 30.2 g (0.2 mol) of p-methylaminobenzoic acid was mixed with 280 ml of 1,2-dichloroethane at room temperature and stirred for 1 hour, then 33.6 g (0.4 mol) of sodium bicarbonate was added and cooled to -20 o C, slowly add 37.4 g (0.21 mol) of N-bromosuccinimide, keep the low temperature and continue the reaction for 5 hours, finish the reaction with 50 mL of saturated aqueous sodium thiosulfate solution, add 100 mL of ethyl acetate, separate the organic phase, Then wash with 300 mL of saturated sodium chloride aqueous solution, dry over anhydrous sodium sulfate, concentrate under reduced pressure to about 100 mL, add 50 mL of n-hexane, a solid precipitates, stir at room temperature for 2 hours, filter with suction, and wash with 50 mL of dichloromethane wash, 50 o C was dried in vacuum for 1 hour to obtain 42.1 g of white powdery solid, yield: 92%.
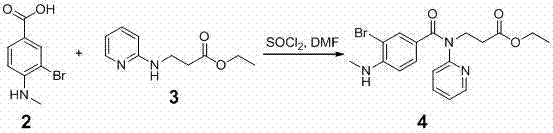
[0059] 2. Preparation of the compound of formula 4
[0060] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com