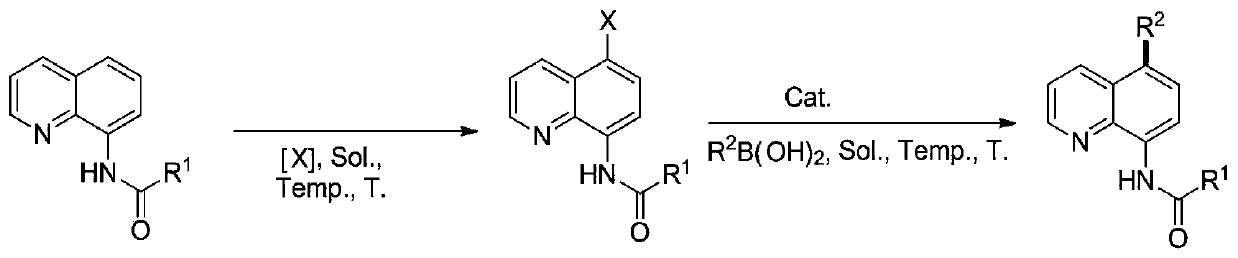
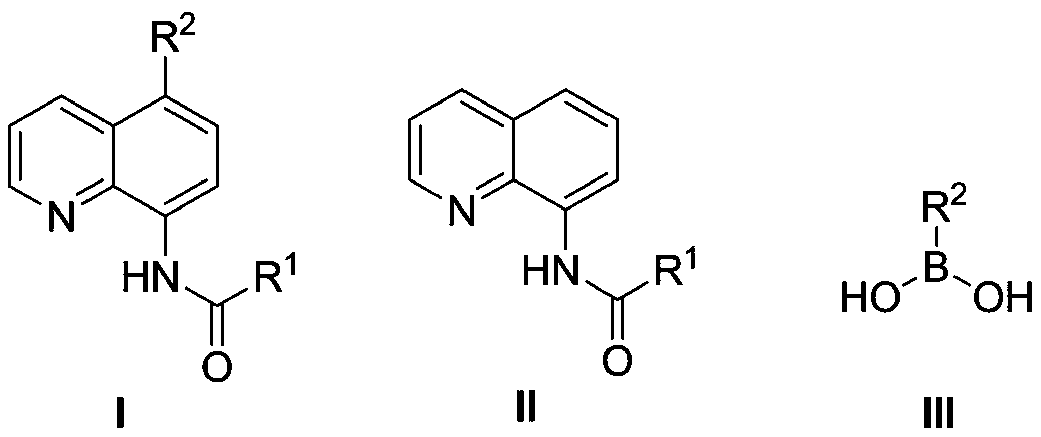
A kind of 5-substituted arylation/heterocyclic 8-amidoquinoline compound and its one-pot preparation method
A technology of quinoline and arylation, applied in the field of catalytic organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0019] Add 0.2mmol 8-(pivaloyl)aminoquinoline (R 1 =tert-butyl), 0.22 mmol NBS and 1 mL DMF, under a nitrogen atmosphere, the reaction was carried out at 160° C. for 24 h. After the completion of the reaction, the reaction was cooled to room temperature, then 0.3mmol phenylboronic acid, 0.4mmol sodium carbonate and 0.02mmol catalyst tetrakis (tri (2-methyl) phenyl) phosphine palladium were added under a nitrogen atmosphere, and the solvent dimethyl sulfoxide ( DMSO) (1mL) (1mL), reacted at 140°C for 6 hours, after the reaction, filtered, concentrated, and separated by column chromatography to obtain 5-phenyl-8-(pivaloyl)aminoquinoline, the yield was 83%.
preparation example 2
[0021] Add 0.2mmol 8-(pivaloyl)aminoquinoline (R 1 =Me), 0.22 mmol NIS and 1 mL DMF, under a nitrogen atmosphere, the reaction was carried out at 160° C. for 24 h. After the reaction was completed, the reaction was cooled to room temperature, then 0.3mmol phenylboronic acid, 0.4mmol sodium carbonate and 0.02mmol catalyst tetrakis (tri (4-methyl) phenyl) phosphine palladium were added under a nitrogen atmosphere, and the solvent dimethyl sulfoxide ( DMSO) (1mL), reacted at 140°C for 6 hours, after the reaction, filtered, concentrated, and separated by column chromatography to obtain 5-phenyl-8-(methylacetamido)quinoline with a yield of 78% .
preparation example 3
[0023] Add 0.2mmol 8-(pivaloyl)aminoquinoline (R 1 =n-octly), 0.22 mmol NCS and 1 mL DMF, under a nitrogen atmosphere, the reaction was carried out at 160° C. for 24 h. After the reaction was completed, the reaction was cooled to room temperature, then 0.3mmol phenylboronic acid, 0.4mmol sodium carbonate and 0.02mmol catalyst tetrakis(tri(4-tert-butyl)phenyl)phosphine palladium were added under a nitrogen atmosphere, and the solvent dimethyl sulfoxide was added (DMSO) (1mL), reacted under the environment of 140 ℃ for 6 hours, after the reaction was finished, filtered, concentrated, separated by column chromatography to obtain 5-phenyl-8-(n-octylacetamido) quinoline, the yield was 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com