Preparation and Application of a Novel Fluorescent Probe for Specific Recognition of Cysteine
A cysteine and fluorescent probe technology is applied in the application field of fluorescent probes for detecting cysteine in vitro and in living cells, and can solve the problems of large Stokes shift and good penetration, etc. Achieving the effects of large Stokes shift, good selectivity and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of intermediate product
[0019] Dissolve 1,4-diethyl-1,2,3,4-tetrahydroquinoxaline salicylaldehyde (0.1172g, 0.5mmol) in 5mL of anhydrous dichloromethane, then add triethylamine (0.14ml , 1.0mmol) and acryloyl chloride (0.1358g, 1.5mmol), protected by argon, reacted for 1h under stirring at room temperature, quenched the reaction with distilled water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and removed dichloromethane by rotary evaporation. Separation by column chromatography yielded a brown oil. Yield: 0.1261 g. Yield: 87.5%. 1 H NMR (500MHz, CDCl3)δ H 9.84(s,1H),6.97(s,1H),6.64(dd,J=17.3,1.2Hz,1H),6.37(dd,J=17.3,10.5Hz,2H),6.21(s,1H),6.08 -6.01(m,1H),3.51(t,2H),3.40-3.35(m,4H),3.24(t,2H),1.22-1.17(m,6H). 13 C NMR (125MHz, CDCl3)δ C 186.1, 164.9, 147.6, 142.0, 132.9, 132.3, 127.8, 116.5, 108.7, 102.5, 47.4, 45.8, 45.3, 44.6, 10.7, 10.0.
Embodiment 2
[0020] Embodiment 2: the synthesis of probe molecule
[0021] The product obtained in the previous step (0.0864g, 0.3mmol) was dissolved in 7mL of anhydrous dichloromethane, triethylamine (0.042mL, 0.3mmol) was added, and malononitrile (0.0198g, 0.3mmol) was added. , Stir at room temperature for 0.5h, quench the reaction with distilled water, extract with dichloromethane, dry over anhydrous sodium sulfate, spin evaporate the solvent, obtain the product by column chromatography, dry in vacuo overnight to obtain a red solid. Yield: 0.0108 g. Yield: 10%. The structure of the probe molecule is characterized as follows: 1 H NMR (500MHz, CDCl3)δ H 7.56(d, J=2.6Hz, 2H), 6.68(dd, J=17.3, 0.9Hz, 1H), 6.38(dd, J=17.3, 10.5Hz, 1H), 6.28(s, 1H), 6.14(dd ,J=10.5,0.9Hz,1H),3.60(t,2H),3.44(q,J=7.2,2H),3.39(q,J=7.2,2H),3.29(t,2H),1.24(t ,6H). 13 C NMR (125MHz, CDCl3)δ C 164.2, 149.8, 146.7, 143.4, 134.0, 132.6, 127.1, 116.6, 115.1, 112.2, 106.8, 102.5, 70.9, 47.7, 46.0, 45.6, 44.4, 1...
Embodiment 3
[0022] Embodiment 3: The present invention: the application of fluorescent probe
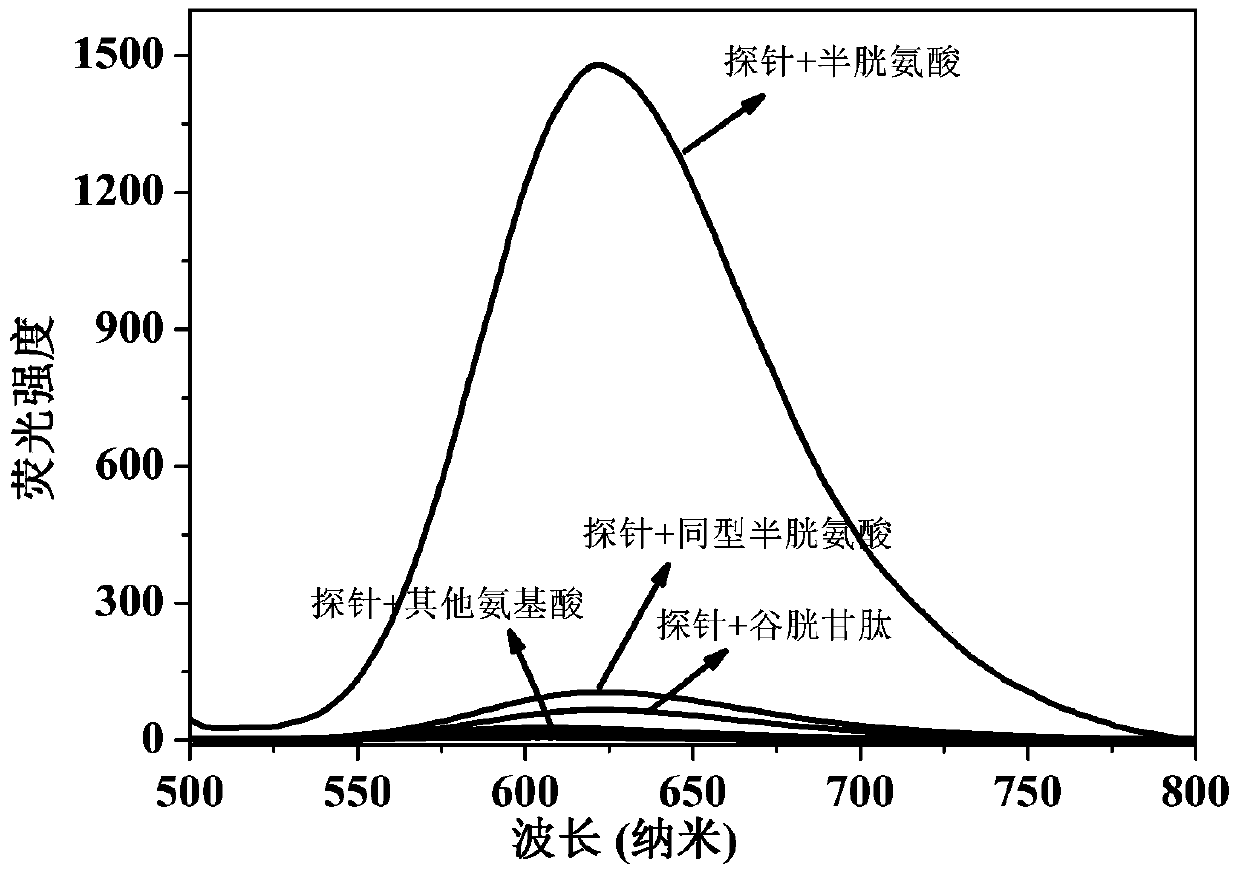
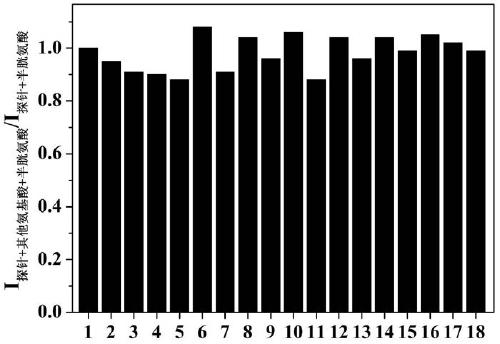
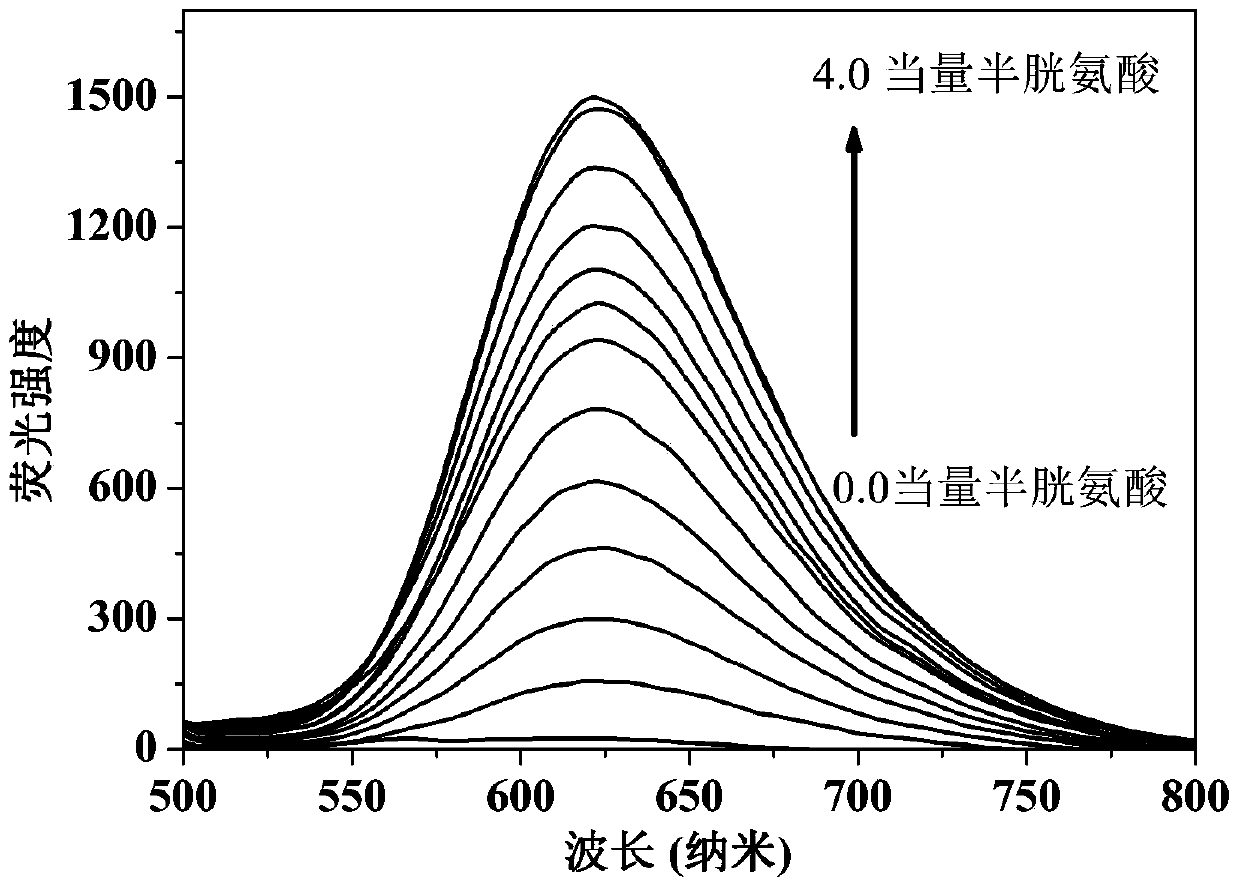
[0023] Dissolve the probe in the buffer solution (V 乙腈 / V PBS =2 / 8, pH=7.4) to prepare 1.0×10-5 mol / L solution, adding amino acids (Ala, Val, Try, Phe, His,, Iso, Ser, Asp, Lys, Arg, Gly, Met, Tyr, Glu, Thr) to the solution did not cause a change in fluorescence, adding Human amino acids (Cys, Hcy, GSH) cause fluorescence changes, and this fluorescent probe shows high sensitivity and high selectivity for cysteine recognition. When cysteine coexists with interfering substances (Ala, Val, Try, Phe, His,, Iso, Ser, Asp, Lys, Arg, Gly, Met, Tyr, Glu, Thr, Hcy, GSH), the probe does not Affected by interference factors, it shows a strong anti-interference ability. The probe molecule responds quickly to cysteine, and the change of fluorescence can be observed within 1 minute. The probe molecule can selectively recognize cysteine in the range of pH 7 to 11, showing the application range of bio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com