A kind of preparation method of Zolpidem
A technology for zolpidem and pyridine, which is applied in the field of preparation of zolpidem, can solve the problems of industrial production limitation, low atom utilization rate, long reaction steps, etc., and achieves the effects of reducing pollution, being easy to implement, and having low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
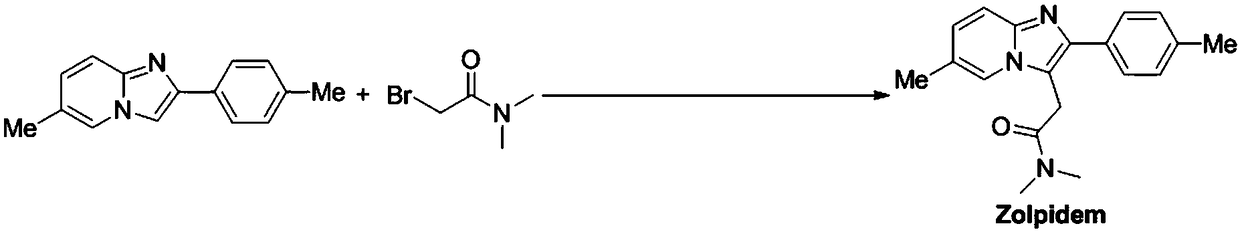
[0020] Under nitrogen protection conditions, add 6-methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine (1.11g, 5mmol) into a 100mL reaction flask, add Ir(ppy) 2 (dtbbpy)PF 6 (65.5mg, 2mol%), 2-bromo-N,N-dimethylacetamide (1.66g, 10mmol), N,N-dicyclohexylmethylamine 2.14mL (1.95g, 10mmol), 30mL acetonitrile as solvent , under the irradiation of a 5W blue LED lamp, stirred at 40°C for 18h, quenched the reaction with water, extracted with dichloromethane (60mL×3), washed with 50mL saturated brine, dried over anhydrous sodium sulfate, and concentrated under vacuum. Purified and separated by column chromatography to obtain white solid N,N,6-methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine-3-acetamide (zolpidem) 0.97 g, the yield is 63%, and the structure detection data of the prepared Zolpidem are as follows:
[0021] m.p.193-195°C (reported m.p.194-196°C); 1 H NMR (400MHz, CDCl 3 ):δ(ppm)7.93(s,1H),7.52–7.49(m,3H),7.22(d,J=7.6Hz,2H),7.03(d,J=8.8Hz,1H),4.03(s, 2H), 2.92(s,3H), 2....
Embodiment 2
[0023] Same as Example 1, the difference is:
[0024] The molar ratio of 6-methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine to 2-bromo-N,N-dimethylacetamide is 1:2, and the reaction The temperature was 20°C and the reaction time was 18 hours.
[0025] The photocatalyst is fac-Ir(ppy) 3 , its dosage is 0.5% (molar ratio) of 6-methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine; the base is sodium bicarbonate, the base and 6-methyl The molar ratio of -2-(4-methylphenyl)-imidazo[1,2-a]pyridine is 2:1; the organic solvent used for extraction is ethyl acetate.
[0026] N,N,6-Methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine-3-acetamide (zolpidem) was isolated as a white solid in 65% yield .
[0027] The structure detection data of the prepared Zolpidem are basically the same as in Example 1.
Embodiment 3
[0029] Same as Example 1, the difference is:
[0030] The molar ratio of 6-methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine to 2-bromo-N,N-dimethylacetamide is 1:4, and the reaction The temperature was 60°C and the reaction time was 12 hours.
[0031] The photocatalyst is Ir(ppy) 2 (dtbbpy)PF 6 , its dosage is 2.8% (molar ratio) of 6-methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine; the base is sodium acetate, the base and 6-methyl- The molar ratio of 2-(4-methylphenyl)-imidazo[1,2-a]pyridine is 2:1.
[0032] N,N,6-Methyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine-3-acetamide (zolpidem) was isolated as a white solid in a yield of 66% .
[0033] The structure detection data of the prepared Zolpidem are basically the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com