Isoindole bora fluorescent dye and its preparation method and application
A fluorescent dye, isoindole boron technology, applied in the field of fluorescent dyes, can solve the problems of poor spectral selectivity, cumbersome preparation process, low quantum yield, etc. The effect of easy availability of raw materials and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
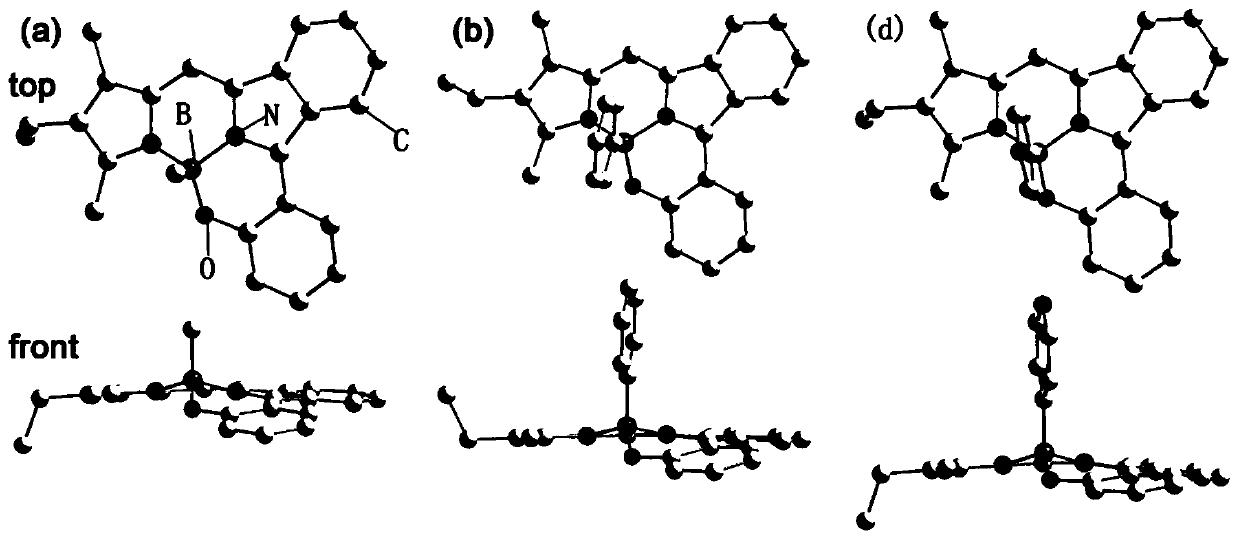
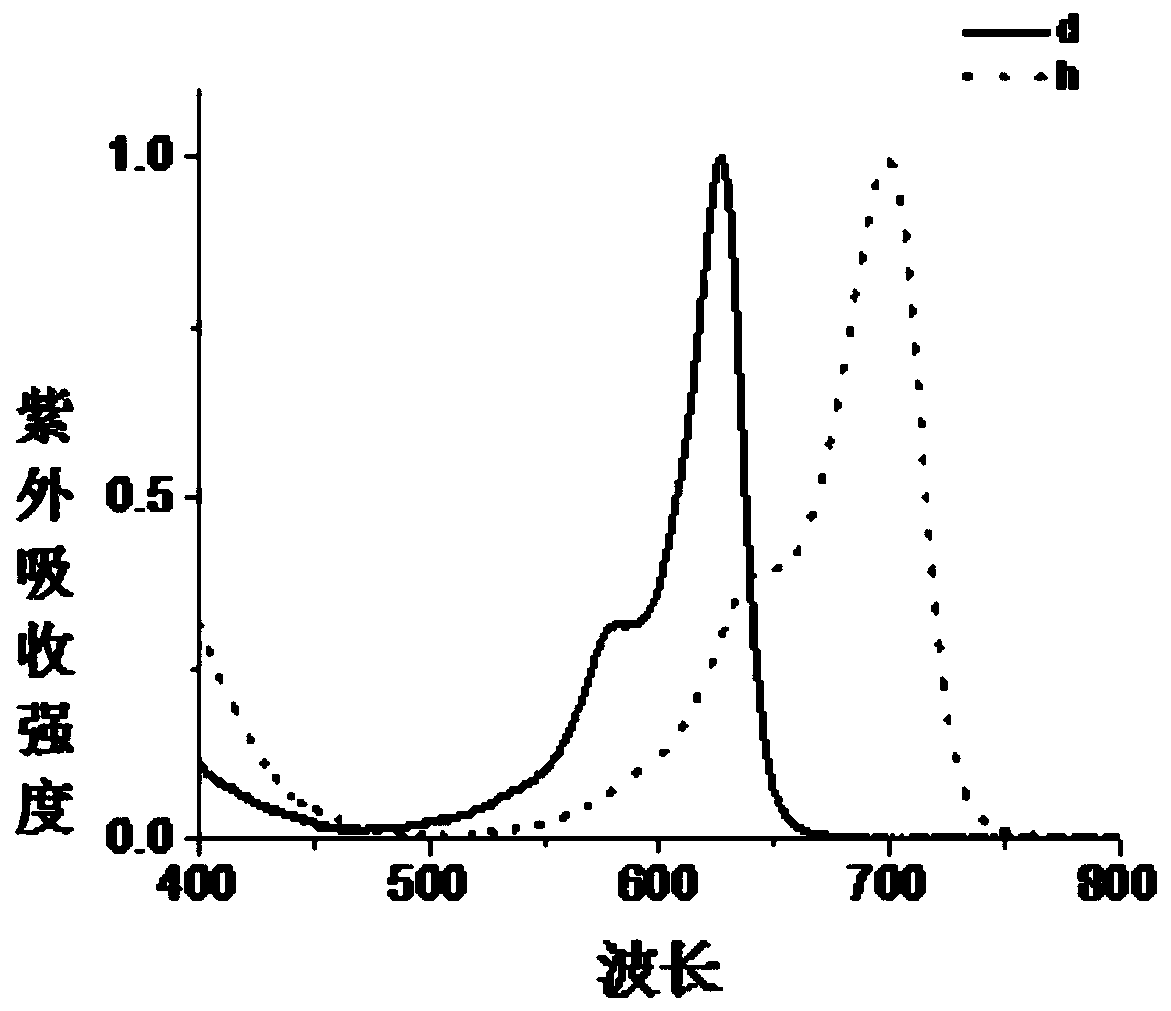
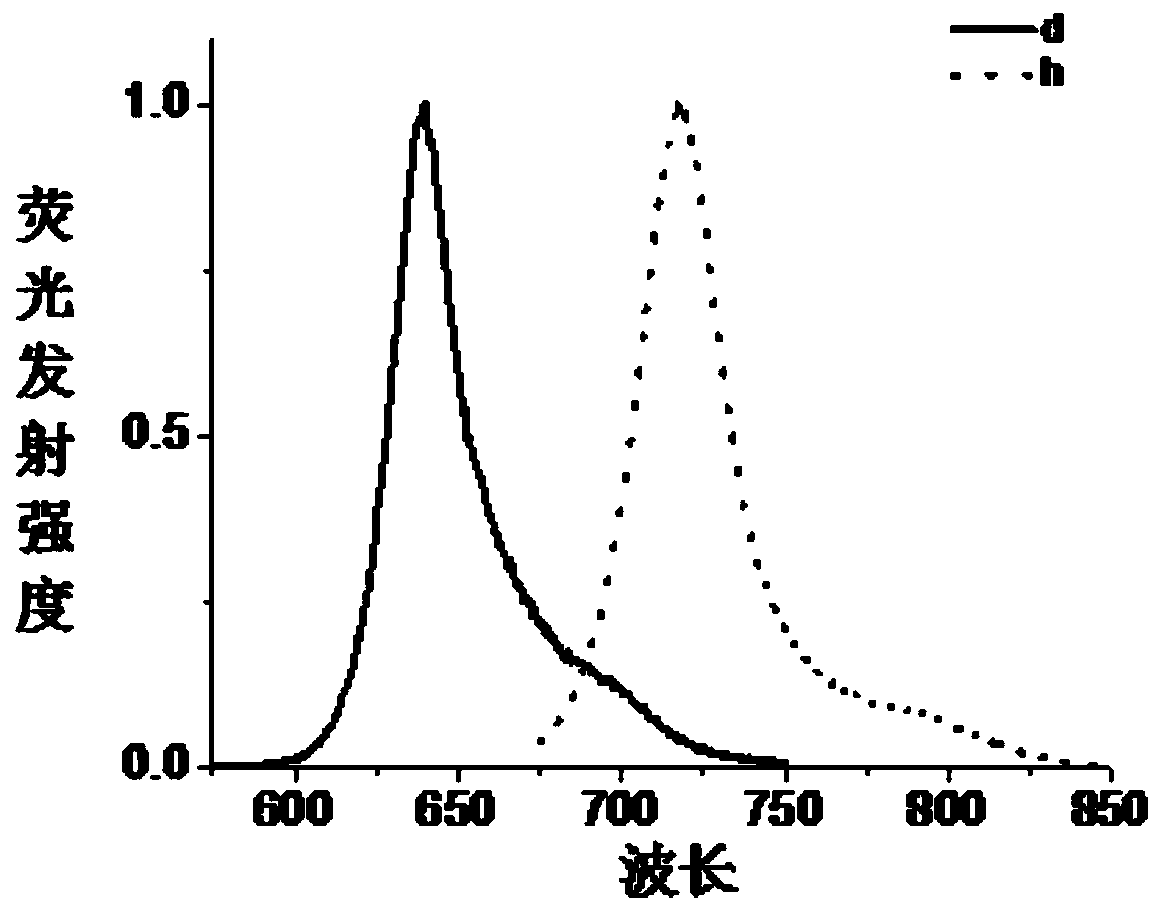
Image
Examples
preparation example Construction
[0050] The present invention also provides a method for preparing the above-mentioned isoindole bora fluorescent dye, wherein the preparation method comprises the following steps:
[0051] 1) In the presence of a solvent and a Lewis acid, a first contact reaction is carried out between a compound shown in formula (II) and a compound shown in formula (III) to obtain a first product;
[0052] 2) In the presence of a solvent, add a boronic acid compound with a structure shown in formula (X) to the first product to carry out a second contact reaction to obtain the isoindole bora fluorescence as shown in formula (I) dye;
[0053]
[0054] Wherein, R1, R2, R3, R4 are each independently hydrogen, C1-C12 alkyl, halogen or C1-C12 alkoxy;
[0055] R5, R6, R7, R8 are each independently hydrogen, C1-C12 alkyl, halogen, C1-C12 alkoxy, cycloalkyl or aryl;
[0056] R9 is hydrogen, alkenyl or C1-C12 alkyl;
[0057] R10 and R11 are each independently hydrogen, C1-C12 alkyl, halogen or al...
preparation example 1
[0077] Preparation of raw material 1-(3-bromo-1H-isoindol-1-ylidene)-N,N-dimethylmethylamine: 1.5g (5.24mmol) POBr 3 Place in a 250ml round bottom flask, add 50ml of dichloromethane, cool to 0°C, add 0.27ml (3.49mmol) DMF dropwise to the solution under stirring, drop it within 5min, then place the reaction at 20°C and stir After 0.5 hours, cool to 0° C., weigh 1.0 g (7.5 mmol) of isoindol-1-one, dissolve it in dichloromethane, place it in a constant-pressure dropping funnel, and add it dropwise to the above mixture. After the dropwise addition was completed, it was transferred to an oil bath at 60°C and heated to reflux, and the plate was spotted by TLC. After 2.5 hours, the reaction is complete. After cooling to 25°C, add 100ml of ice water, adjust the pH value to 8 with sodium carbonate, extract, concentrate, pass through a column, and recrystallize to obtain a light yellow solid.
preparation example 2
[0079] Preparation of raw material (2-hydroxyphenyl)-2H-isoindole-1-carbaldehyde: Weigh 1-(3-bromo-1H-isoindole-1-ylidene)-N,N-dimethylformaldehyde Amine 400mg (1.66mmol), 2-hydroxyphenylboronic acid 331mg (3.5mmol) Na 2 CO 3 (1M, 5mL) in a 100ml Schlenk reactor, add Pd(PPh 3 ) 4 60mg, (0.05mmol), after flushing three times and releasing three times, moved to a 75°C oil bath and heated and stirred for 12h. After cooling to 25°C, extract with water, collect the organic phase, dry over anhydrous sodium sulfate, and rotary evaporate with a rotary evaporator to obtain a yellow solid. After dissolving with absolute ethanol, add saturated sodium hydroxide solution and heat to reflux for 3h. After removing ethanol, extract with water and ethyl acetate, neutralize with dilute HCl (3M) to pH 7, collect the organic phase, dry over anhydrous sodium sulfate, rotary evaporate with a rotary evaporator, and recrystallize to obtain a yellow solid
[0080] The proton nuclear magnetic spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com