A kind of genetically engineered bacteria and its construction method and method for producing vanillin
A technology of genetically engineered bacteria and vanillin, applied in the field of bioengineering, can solve problems such as the need to improve efficiency, long routes, and cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
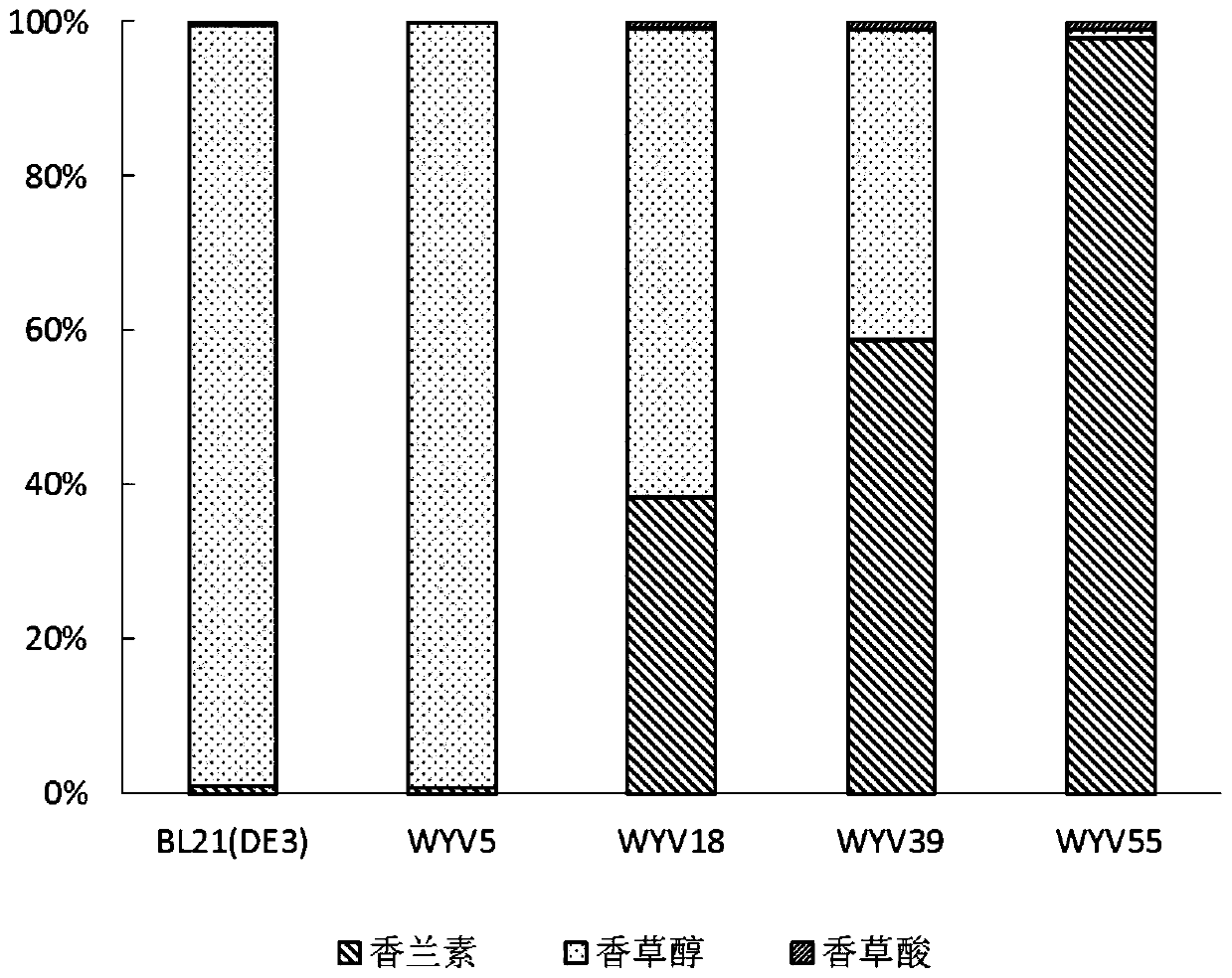
[0056] Example 1: Knockout of shikimate dehydrogenase encoding gene aroE in Escherichia coli BL21(DE3)
[0057] The shikimate dehydrogenase encoding gene aroE in Escherichia coli BL21(DE3) was knocked out by PCR Targeting technology, and the obtained strain was named WYV5. The specific operation is as follows:
[0058] Using the BL21(DE3) strain genome as a template, the primer pairs aroE_up_s, aroE_up_a and aroE_dw_s, aroE_dw_a were used to amplify the upstream and downstream homology arms of the aroE gene, and pKD3 was used as a template to amplify the chloride The mycin resistance gene fragment, the three fragments were amplified by overlapping PCR to obtain the aroE gene knockout fragment aroE::cm, the primer sequences are as follows:
[0059] aroE_up_s: 5'-AACGGAAGCCGTTTTCGGTG-3';
[0060] aroE_up_a: 5'-CATTATGTTACCCCTGTCGA-3';
[0061] aroE-cm_s: 5′-AATCCGCGATGCCCTGACGGGTGAACTGTTTCGACAGGGGTAACATAATGGTGTAGGCTGGAGCTGCTTC-3′;
[0062] aroE-cm_a: 5′-ATTCTCGTCCCACTCTTCCCT...
Embodiment 2
[0073] Example 2: Knockout of a possible vanillin-degrading gene cluster yqhC-yqhD-dkgA
[0074] Using PCR Targeting technology, the possible vanillin degradation gene cluster yqhC-yqhD-dkgA was knocked out of the strain obtained in Example 1, and the obtained strain was named WYV18. The specific operation is as follows:
[0075] Using the BL21(DE3) strain genome as a template, the primer pairs yqhC-dkgA_up_s, yqhC-dkgA_up_a and yqhC-dkgA_dw_s, yqhC-dkgA_dw_a were used to amplify the upstream and downstream homology arms of the yqhC-yqhD-dkgA gene cluster, and pKD3 was used as a template, Amplify the chloramphenicol resistance gene fragment with primer pair yqhC-dkgA-cm_s, yqhC-dkgA-cm_a, three fragments are obtained yqhC-yqhD-dkgA gene cluster knockout fragment yqhC-yqhD-dkgA by overlapping PCR amplification: :cm. The primer sequences are as follows:
[0076] yqhC-dkgA_up_s: 5'-TTTTCTGCCTACGATTGC-3';
[0077] yqhC-dkgA_up_a: 5'-GGAAAATCGTCAGGCGTTAC-3';
[0078] yqhC-dkgA...
Embodiment 3
[0089] Example 3: Knockout of a possible vanillin degradation gene yahK
[0090] Using the PCR Targeting technique, the possible vanillin degradation gene yahK was knocked out of the strain WYV18 obtained in Example 2, and the obtained strain was named WYV39. The specific operation is as follows:
[0091] Using the BL21(DE3) strain genome as a template, the primer pair yahK_up_s, yahK_up_a and yahK_dw_s, yahK_dw_a were used to amplify the upstream and downstream homology arms of the yahK gene, and pKD3 was used as a template to amplify the chloride Mycin resistance gene fragments, the three fragments were amplified by overlapping PCR to obtain the yahK gene knockout fragment yahK::cm, the primer sequences are as follows:
[0092] yahK_up_s: 5'-AGATGAACTGGCGAACCGGA-3';
[0093] yahK_up_a: 5′-GAACTTCGAAGCAGCTCCAGCCTACACCATTGTGTTTACTCCTGATTAGC-3′;
[0094] yahk-cm_s: 5′-GCTAATCAGGAGTAAACACAATGGTGTAGGCTGGAGCTGCTTCGAAGTTC-3′;
[0095] yahK-cm_a: 5'-TGTTAAACCACAGGGTATTTTATTAATTT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com