Compound separated from colletotrichum gloesporioides and preparation method and application thereof
A technology of anthracnose and compounds, which are applied in biochemical equipment and methods, organic chemistry methods, microorganism-based methods, etc., to achieve the effects of improving learning and memory ability, easily controllable preparation conditions, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of compounds of the present invention:
[0036] (1) Fermentation of Glosporum anthracnose
[0037] a. Strain activation: Take the frozen strain of G. anthracis, inoculate it on the plate of PDA solid medium in the ultra-clean workbench, and cultivate it in a constant temperature incubator at 28°C for 7 days;
[0038] b. Inoculation and primary fermentation of strains: inoculate the activated strains into 500ml Erlenmeyer flasks containing 200mL PDB liquid medium, a total of 12 flasks were placed in a rotary shaker in a constant temperature air bath at 28°C. The rotation speed is 160r / min, the culture time is 10 days, and the total fermentation volume is 2.4L;
[0039] c. Large-scale fermentation of strains: prepare a total of 45L of PDB liquid medium, sterilize it on a steam generator, the sterilization temperature is 115-118°C, and the sterilization time is 2.5h. After the sterilization is completed, cool the medium to room temperature Dissolve one bottle...
Embodiment 2
[0047] Determination and verification of the structural formula of the compound of the present invention:
[0048] (1) Physicochemical property data of the compound
[0049] Colorless oil, optical rotation: 30; the developing system is petroleum ether:acetone=2:1, and the color of sulfuric acid ethanol is light yellow spots.
[0050] (2) Determination of the molecular formula of the compound
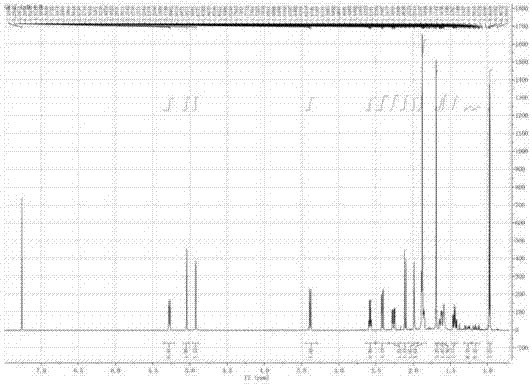
[0051] combine 1 H-NMR and 13 C-NMR data and HRESIMS (found value 273.1466 [M+Na] + , calculate C 15 H 22 O 3 The Na value is 273.1461), and its molecular formula is determined to be C 15 H 22 O 3 .
[0052] (3) Determination of compound structural formula
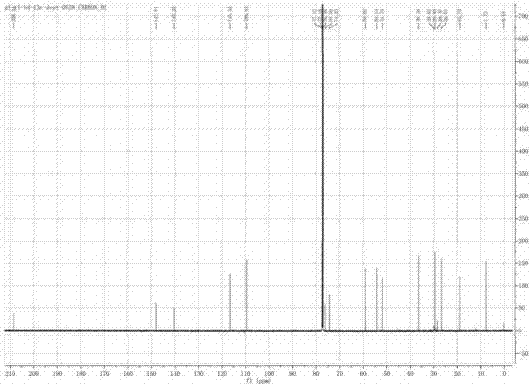
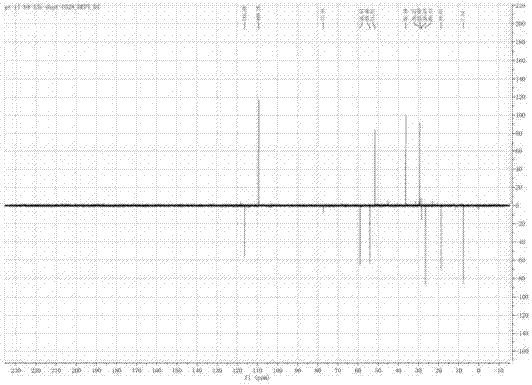
[0053] like figure 2 As shown, the IR spectrum data shows that the compound contains hydroxyl and carbonyl groups (νmax=3406,1724cm -1 ). 1 H-NMR, 13 C-NMR and DEPT spectra (eg Figure 3-Figure 5 shown) shows three methyl groups, three sp3 methylene groups, one sp2 methylene group, two sp3 methine groups, one sp2 met...
Embodiment 3
[0064] Acetylcholinesterase inhibitory activity test of the compounds of the present invention:
[0065] (1) Measurement of acetylcholinesterase inhibitory activity of the compounds of the present invention
[0066] Adopt the modified Ellman method to measure the acetylcholinesterase inhibitory activity of the compound anthraxine A, and Huperzine A is used as the positive control drug, and the specific implementation steps are as follows:
[0067] The modified Ellman method was used for determination. The operation steps were as follows: 140 μL PBS (0.1M pH=8.0), 20 μL sample solution (final concentration: 1 mg / mL), 15 μL AChE (0.28 U / mL, 15 μL AChE (0.28 U / mL, pH=8.0 PBS solubilization dilution). After incubation at 4°C for 20 min, 10 μL DTNB (0.075 mol / L) and 10 μL ATCI (0.01 mol / L) were added. Incubate at 37 °C for 20 min, and measure the absorbance at 405 nm with a microplate reader. Among them, the blank group used 20 μL PBS (pH 8.0) to replace 20 μL of sample solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com