Aptamer-based rapid kanamycin detection test paper as well as preparation method and application thereof
A nucleic acid aptamer, kanamycin technology, applied in measurement devices, instruments, scientific instruments and other directions, can solve the problems of long time, high operator requirements, high cost, easy preparation and modification, improve detection efficiency, The effect of short detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
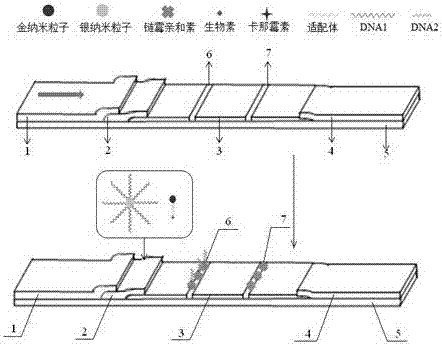
[0040] Example 1 A rapid detection test paper for kanamycin based on nucleic acid aptamer
[0041] Preparation and functionalization of gold nanoparticles, preparation and functionalization of silver nanoparticles, pretreatment of gold standard pads and sample pads, pretreatment of nitrocellulose membranes, assembly of test strips, and detection of color development of test strips. The specific steps are:
[0042] (1) Preparation and functionalization of gold nanoparticles:
[0043] Before preparing gold nanoparticles, all glass instruments were soaked in aqua regia for 30min, then washed with distilled water, soaked in ultrapure water for 12h, and dried for later use; 100mL of 0.01% chloroauric acid was added to a 250mL round bottom flask, stirred and heated To boiling, quickly add 3.5mL of 1% trisodium citrate under vigorous stirring, continue to heat and stir for 15 minutes, and the solution turns wine red, stop heating, continue to stir for 30 minutes, let it stand, and c...
Embodiment 2
[0056] Embodiment 2 Determination of kanamycin standard solution with test strips
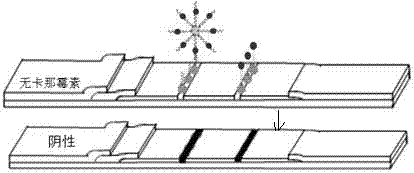
[0057] Use 2mL centrifuge tubes to dilute kanamycin to different concentrations, 100 µL per tube. Insert one end of the sample pad of the test strip prepared by the above method into the standard solution of kanamycin with different concentrations, and react at room temperature for 10 min. From Figure 4 It can be seen that the color of the T line basically does not change between 0-1 nmol / L and 35-400 nmol / L of kanamycin concentration, while the color of the T-line changes with the concentration of kanamycin between 1-35 nmol / L. It becomes lighter with the increase of the element concentration, and the detection line basically has no color after 35 nmol / L. Therefore, the naked eye detection limit of the test paper for kanamycin was 35 nmol / L. Put the reacted test strip into a plastic card and use a colloidal gold quantifier to detect the color development of the T and C lines, and obtain th...
Embodiment 3
[0058] Example 3 Detection of Kanamycin Residues in Milk Samples
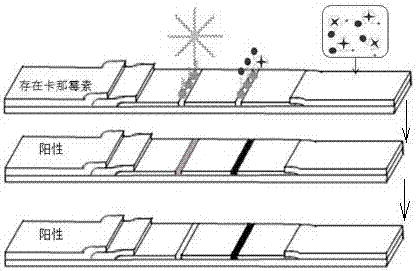
[0059] Here, artificially contaminated milk was prepared by adding standard concentration of kanamycin to milk to obtain 5 nmol / L, 10 nmol / L, 20 nmol / L, 30 nmol / L, 50 nmol / L, 100 nmol / L , 200 nmol / L kanamycin milk samples. Add 20% trichloroacetic acid dropwise to the milk sample, adjust the pH to 4.6, and precipitate casein in a water bath at 45°C for 10 minutes, centrifuge at 10,000 r / min for 25 minutes to remove coagulated protein and fat, and filter with a 0.22 μm filter membrane , and finally adjust the pH to neutral to obtain the pretreated milk sample. The milk samples containing different concentrations of kanamycin were detected according to the above operation steps. get Figure 6 The test strips of milk samples containing different concentrations of kanamycin are shown. exist Figure 6 It can be seen from the figure that as the concentration of kanamycin increases, the color of the T line becomes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com