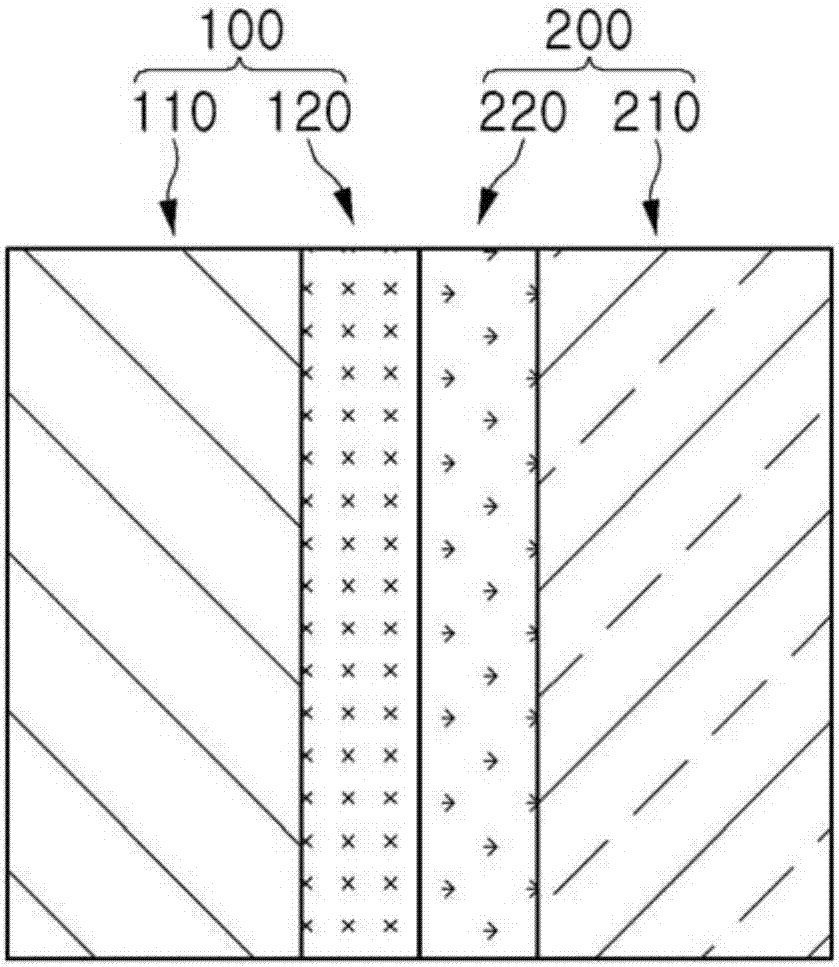
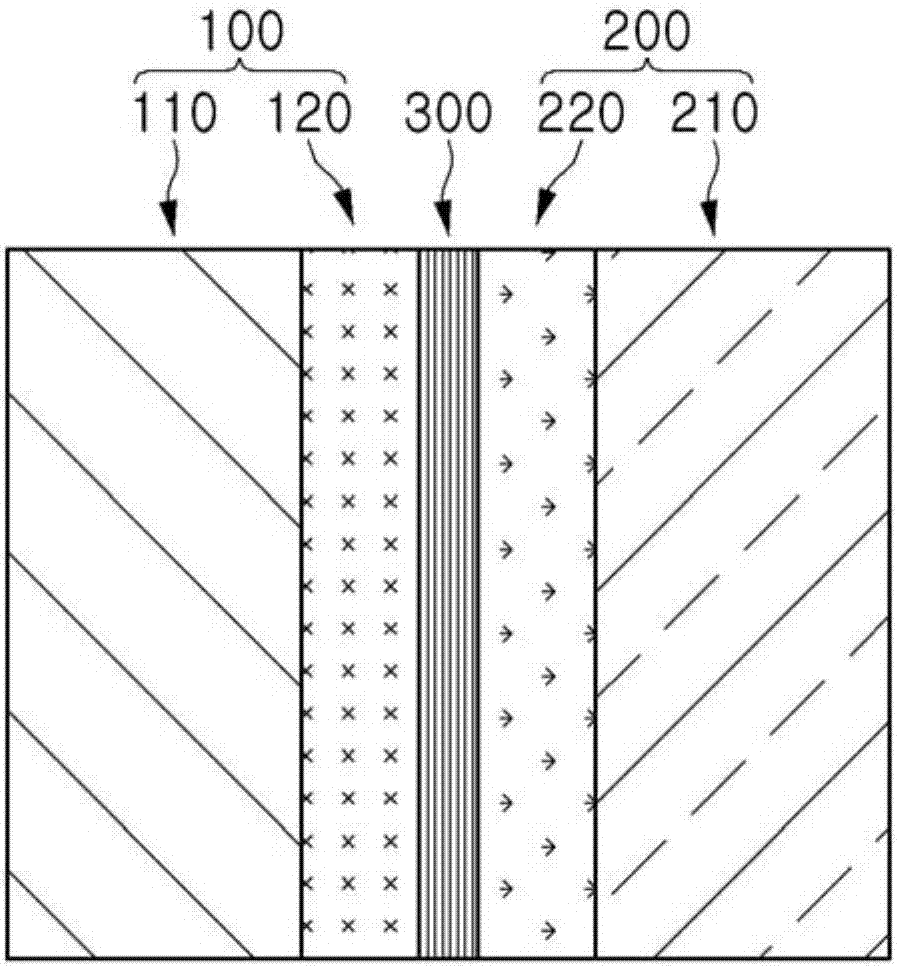
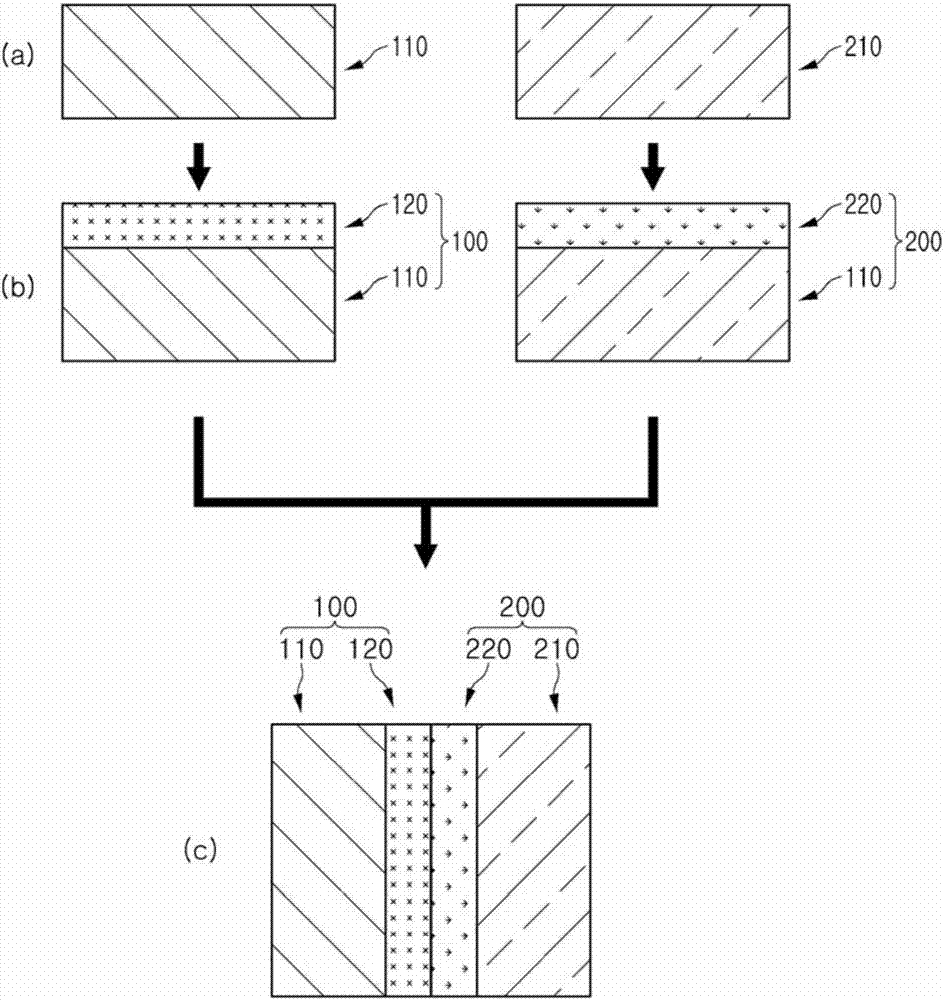
Bipolar ion exchange sheet and manufacturing method therefor
A technology of ion exchange and cation exchange, applied in bipolar ion exchange plate and its manufacturing field, can solve the problems of water splitting efficiency drop, high membrane resistance, low ion selectivity, etc., achieve improved regeneration efficiency, low membrane resistance, increase ion The effect of swap capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0088] Manufacturing example 1: Manufacturing of porous cation adsorption plate
[0089] To fabricate porous cation adsorption sheets, cation exchange resin (Trilite CMP28, Samyang Corporation) was pulverized with an air jet mill (model name: NETSCH-CONDUX, NETSCH Corporation) at a rotation speed of 1,700 RPM to A cation exchange resin powder having an average particle diameter of 2.5 μm (maximum 10.2 μm) was prepared.
[0090] The mixture was produced by using 40 wt% LLDPE powder with an average particle size of 400 [mu]m to 60 wt% cation exchange resin powder using a ball mill. At this point, the LLDPE and cation exchange resin powders were mixed for 24 hours using zirconium balls and polyethylene (plastic) jars.
[0091] The porous cation adsorption plate was manufactured in the following manner: a stainless steel spacer (spacer) with a 20cm x 20cm internal space and a thickness of 200 μm was placed between two stainless steel plates with a size of 25cm x 25cm, and 6g wa...
manufacture example 2
[0092] Manufacturing example 2: Manufacturing of porous anion adsorption plate
[0093] In order to fabricate porous anion adsorption sheets, anion exchange resin (Trilite AMP28, Samyang Corporation) was pulverized with an air jet mill (model name: NETSCH-CONDUX, NETSCH Corporation) at a rotation speed of 1,700 RPM to Anion exchange resin powder having an average particle diameter of 3.7 μm (maximum 13.1 μm) was prepared.
[0094] The mixture was produced by using 40 wt% LLDPE powder with an average particle size of 400 [mu]m to 60 wt% anion exchange resin powder using a ball mill. At this point, the LLDPE and anion exchange resin powders were mixed for 24 hours using zirconium balls and polyethylene (plastic) jars.
[0095] A porous anion adsorption plate was fabricated by placing stainless steel spacers (spacers) with an inner space of 20 cm x 20 cm and a thickness of 200 μm between two stainless steel plates of size 25 cm x 25 cm, and distributing 6 g evenly between the ...
manufacture example 3
[0096] Manufacturing example 3: Formation of a cation exchange coating on a porous cation adsorption plate
[0097] Based on 100 parts by weight of polyetheretherketone (PEEK) (450PF, Dict, South Korea), PEEK was dissolved in 10 parts by weight of sulfuric acid (98%) in a round-bottomed four-neck flask, and then at 80 ° C under a nitrogen atmosphere, The sulfonation reaction was carried out for 24 hours while stirring, resulting in the precipitation of the polymer at -5°C with the addition of distilled water.
[0098] The cation exchange resin formed by sulfonated PEEK was manufactured by repeating three times the following process: drying the precipitated polymer in a vacuum oven at 80 °C, dissolving the precipitated polymer in dimethylacetamide (DMAc), and The precipitated polymer was reprecipitated in methanol to remove residual sulfuric acid, and the obtained precipitated polymer was dried.
[0099] A 20 wt% solids cation exchange coating solution was made by adding dry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com