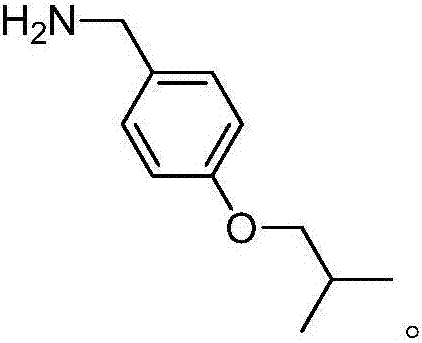
Synthesis method for pimavanserin intermediate
A technology of pimavanserin and synthetic method, which is applied in the field of drug synthesis, can solve the problems of danger, no advantage, and high price in the scale-up production of lithium tetrahydrogen, and achieve good industrial application value, mild reaction conditions, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
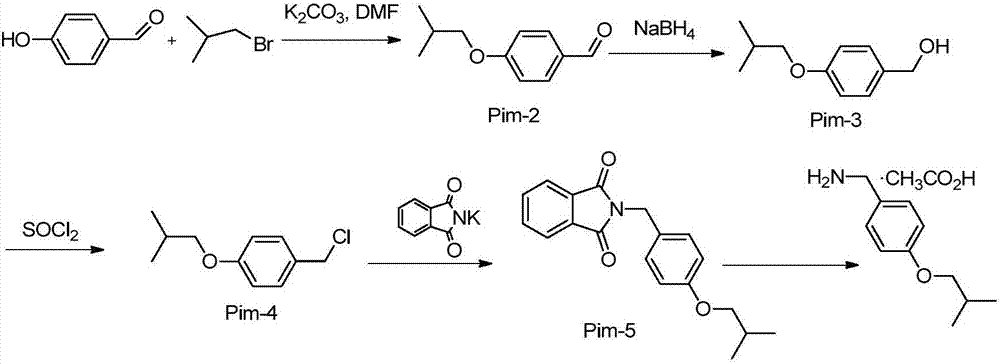
[0028] Example 1 Synthesis of 4-isobutoxybenzaldehyde:
[0029] Add 122g of 4-hydroxybenzaldehyde (1mol), 600mL of DMF, 256g of potassium carbonate (1.85mol) and 12g of potassium iodide (0.07mol) into the reaction flask, then raise the temperature to 100°C, and start to add 274g of bromoisobutane dropwise. After completion, react overnight at 100°C, TLC shows that the reaction of 4-hydroxybenzaldehyde is complete and can be processed, the reaction solution is suction filtered, washed with 600mL of dichloromethane, the filtrate is washed twice with 600mL of water, once with 600mL of saturated saline, and separated The organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated to dryness to obtain 178 g of the product, which was directly put into the next step reaction.
Embodiment 2
[0030] Example 2 Synthesis of 4-isobutoxybenzyl alcohol:
[0031] Dissolve 178g of 4-isobutoxybenzaldehyde (1mol) obtained in the above steps with 900mL of methanol, cool down to 0°C, then add 38g of sodium borohydride (1mol) in batches, control the temperature of the reaction temperature below 10°C, and complete the addition. Raise the temperature to 25°C for 2 hours, TLC monitors that the reaction is complete, add dropwise 6N hydrochloric acid to quench, spin dry, add 900mL dichloromethane and 900mL water, stir, separate the organic phase, wash the organic phase with 900mL saturated saline, and separate the organic phase , dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 180 g of the product, which was directly put into the next reaction.
Embodiment 3
[0032] Example 3 Synthesis of 4-isobutoxybenzyl chloride:
[0033] Dissolve 180g of 4-isobutoxybenzyl alcohol (1mol) obtained in the above steps in 900mL of dichloromethane, add 18g of DMF, cool down to 0°C, add 357g of thionyl chloride (3mol), and heat up to 25 After reacting overnight at ℃, the reaction was complete as monitored by TLC, and 900 mL of water was added dropwise. After dropping, the organic phase was separated, washed with 900 mL of saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 199 g of the product, which was directly put into the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com