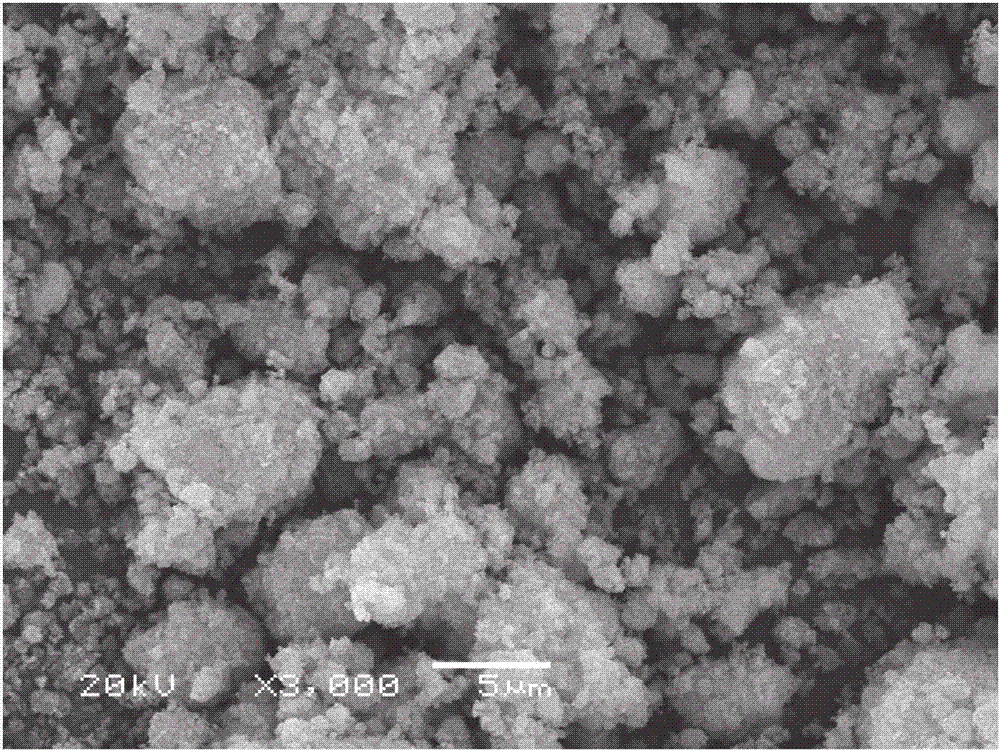
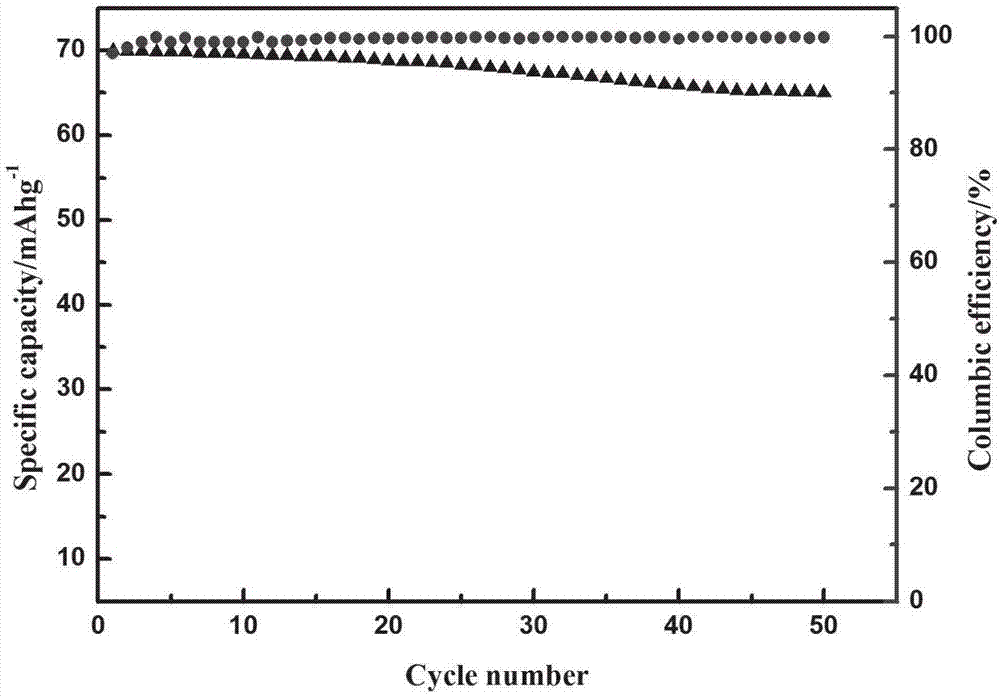
Sodium vanadium pyrophosphate/carbon composite positive electrode material, and preparation and application thereof
A composite cathode material, sodium vanadium pyrophosphate technology, applied in nanotechnology for materials and surface science, battery electrodes, electrical components, etc., can solve the problems of low electronic conductivity, large capacity attenuation, etc., to achieve good repeatability , the effect of low cost and good rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] First weigh 3.5g of aniline (0.045mol), 2g of vanadium pentoxide (0.02mol V) and dissolve in a mixed solution of 100ml of deionized water and ethanol, stir to form a uniform solution, then add 1g of sodium persulfate (0.0042mol) , pour the aqueous solution into a polytetrafluoroethylene reactor, then put the polytetrafluoroethylene reactor into a stainless steel hydrothermal kettle and seal it, and finally place the stainless steel hydrothermal kettle in a homogeneous reactor , hydrothermal reaction was carried out at 180°C for 10 hours. Centrifuge the reacted solution, then place it in an oven at 70°C for drying; take 5 g (0.06 mol) of anhydrous sodium acetate, 5 g (0.043 mol) of ammonium dihydrogen phosphate and the above dried hydrothermal product and ball mill for 10 h at a speed of 300 rad / min. The ball-milled product was sintered in a tube furnace at 600°C for 10 h in an argon atmosphere, washed three times with deionized water, washed twice with alcohol, and dr...
Embodiment 2
[0088]First weigh 3.5g of aniline (0.045mol), 2g of vanadium pentoxide (0.02mol V) and dissolve in a mixed solution of 100ml deionized water and ethanol, stir to form a uniform solution, then add 0.1g of hydrogen peroxide (0.003mol ), the aqueous solution is poured into a polytetrafluoroethylene reactor, then the polytetrafluoroethylene reactor is put into a stainless steel hydrothermal kettle and sealed, and finally the stainless steel hydrothermal kettle is placed in a homogeneous reactor , hydrothermal reaction was carried out at 180°C for 10 hours. Centrifuge the reacted solution, then place it in an oven at 70°C for drying; take 5 g (0.06 mol) of anhydrous sodium acetate, 5 g (0.043 mol) of ammonium dihydrogen phosphate and the above dried hydrothermal product and ball mill for 10 h at a speed of 300 rad / min. The ball-milled product was sintered in a tube furnace at 600°C for 10 hours in an argon atmosphere, washed three times with deionized water, washed twice with alc...
Embodiment 3
[0091] First weigh 3.5g of aniline (0.045mol), 2g of vanadium pentoxide (0.02mol V) and dissolve in a mixed solution of 100ml deionized water and ethanol, stir to form a uniform solution, then add 0.05g of hydrogen peroxide (0.0015mol ), the aqueous solution is poured into a polytetrafluoroethylene reactor, then the polytetrafluoroethylene reactor is put into a stainless steel hydrothermal kettle and sealed, and finally the stainless steel hydrothermal kettle is placed in a homogeneous reactor , hydrothermal reaction was carried out at 180°C for 10 hours. Centrifuge the reacted solution, then place it in an oven at 70°C for drying; take 5 g (0.06 mol) of anhydrous sodium acetate, 5 g (0.043 mol) of ammonium dihydrogen phosphate and the above dried hydrothermal product and ball mill for 10 h at a speed of 300 rad / min. The ball-milled product was sintered in a tube furnace at 600°C for 10 hours in an argon atmosphere, washed three times with deionized water, washed twice with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com