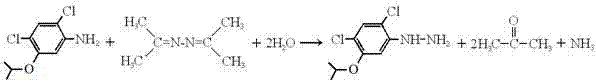
Synthetic process of 2,4-dichloro-5-isopropoxyl phenyl hydrazine
A technology of isopropoxyphenylhydrazine and a synthesis method, which is applied in the fields of hydrazine preparation, organic chemistry and the like, can solve the problems of high equipment requirements, large waste discharge, low yield and the like, and achieves low equipment requirements and waste. The effect of less emission and easy control of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Put 220g of 2,4-dichloro-5-isopropoxyaniline and 168g of acetonazine into a four-neck flask with stirring, dropping funnel, thermometer and rectification column, and the upper part of the receiver of the rectification column is connected to a Conduit to absorb ammonia gas; start stirring, heat the solution to 120-130°C, slowly add 54ml of water dropwise into the four-necked flask, control the reaction temperature at 100°C-130°C, when the gas generated by the reaction enters the rectification column Finally, extract acetone and recover ammonia at the column top temperature of 54°C to 58°C. The water vapor and acetonazine entering the rectification column are condensed and flow back to the flask to continue to participate in the reaction; When no ammonia gas is released from the receiver of the rectifying column, the reaction ends, and all the water and acetonazine in the flask are evaporated with negative pressure. The solid matter in the flask is washed with absolute eth...
Embodiment 2
[0014] Put 220g of 2,4-dichloro-5-isopropoxyaniline and 196g of acetonazine into a four-neck flask with stirring, dropping funnel, thermometer and rectification column, and the receiver of the rectification column is connected to a Conduit to absorb ammonia gas; start stirring, heat the solution to 120-130°C, slowly add 63ml of water into the four-neck flask dropwise, control the reaction temperature at 100°C-130°C, when the gas generated by the reaction enters the rectification column Finally, extract acetone and recover ammonia at the column top temperature of 54°C to 58°C. The water vapor and acetonazine entering the rectification column are condensed and flow back to the flask to continue to participate in the reaction; When no ammonia gas is released from the receiver of the rectifying column, the reaction ends, and all the water and acetonazine in the flask are evaporated with negative pressure. The solid matter in the flask is washed with absolute ethanol and dried to ob...
Embodiment 3
[0016] Put 220g of 2,4-dichloro-5-isopropoxyaniline and 224g of acetonazine into a four-neck flask with stirring, dropping funnel, thermometer and rectification column. Conduit to absorb ammonia gas; start stirring, heat the solution to 120-130°C, slowly add 72ml of water dropwise into the four-neck flask, control the reaction temperature at 100°C-130°C, when the gas generated by the reaction enters the rectification column Finally, extract acetone and recover ammonia at the column top temperature of 54°C to 58°C. The water vapor and acetonazine entering the rectification column are condensed and flow back to the flask to continue to participate in the reaction; When no ammonia gas is released from the receiver of the rectifying column, the reaction ends, and all the water and acetonazine in the flask are evaporated with negative pressure. The solid matter in the flask is washed with absolute ethanol and dried to obtain 2,4-dichloro - 227.2 g of 5-isopropoxyphenylhydrazine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com