A kind of dienyl phosphorescent iridium complex and its preparation method and electroluminescent device
A technology of phosphorescent iridium complexes and alkenyl phosphorescence, applied in the direction of electric solid devices, luminescent materials, electrical components, etc., can solve problems such as impossible applications, and achieve the effects of low production cost, easy implementation, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] A kind of preparation method of the discenyl-containing iridium complex of the present embodiment, add 2.00g (7.09 mmol) 1-formyl triptycene, 0.87g (7.09mmol) o-aminothiophenol and 15mL (194mmol) of anhydrous N,N-dimethylformamide. Heated to 120°C and reacted for 24h under nitrogen atmosphere. After the reaction is complete, add 20 mL of water, extract with ethyl acetate, wash the organic phase with water, dry with anhydrous sodium sulfate and spin dry, use petroleum ether: dichloromethane = 1: 1 to pass through the column, and spin dry to obtain 2-(1- Tridecenyl) benzothiazole ring metallation ligand white solid 2.30g, yield 80%. 1 H NMR (400Hz, CDCl 3 )δ5.64(s, 1H), 7.06(s, 1H), 7.10-7.16(m, 5H), 7.51-7.63(m, 7H), 7.68(t, J=8Hz, 1H), 8.05(d, J=8 Hz, 1H), 8.39 (d, J=8 Hz, 1H).
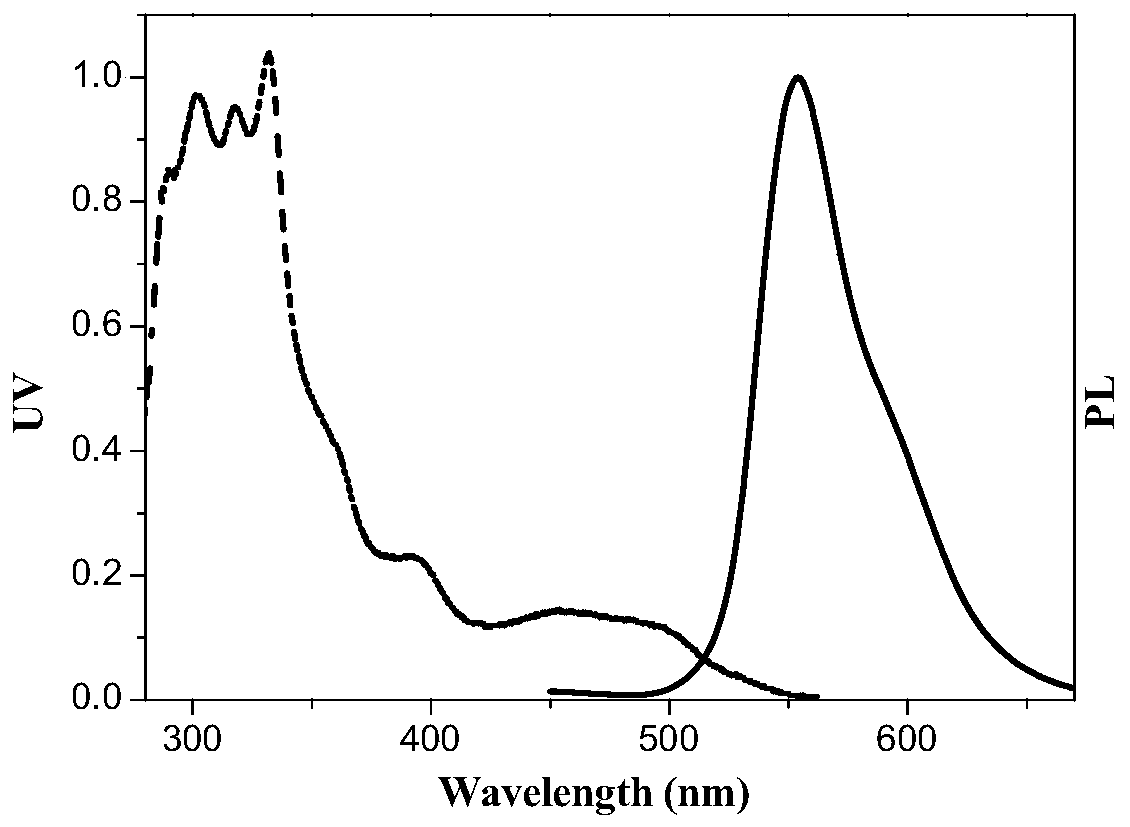
[0066] Take 0.77g (2mmol) 2-(1-triadenyl) benzothiazole, 0.35g (1mmol) iridium trichloride trihydrate into the sealed tube, then add 30mL (310mmol) ethylene glycol ether to the sealed tube ...
Embodiment 2
[0072] A kind of preparation method of the discenyl-containing iridium complex of the present embodiment, 0.20g (0.1mmol) dichloro bridge intermediate, 0.05g (0.4mmol) 2-picolinic acid and 0.14g (1mmol) potassium carbonate are added To the sealed tube, add 20 mL of dichloromethane into the sealed tube, and heat to 60° C. for 5 h. After the reaction is completed, the product is separated by column chromatography to obtain (TPS) 2 Ir(Pic) orange-red solid 0.023g, yield 16% (yield is related to the reaction raw material, different raw material yield is different). (TPS) 2 The NMR spectrum of Ir(Pic) is: 1 H NMR (400Hz, CDCl 3 )δ5.17(s, 1H), 5.27(s, 1H), 5.73(d, J=9.7Hz, 1H), 6.08-6.16(m, 2H), 6.41(d, J=7.8Hz, 2H), 6.66(d, J=7.9Hz, 1H), 6.72(d, J=8.0Hz, 1H), 6.86-7.10(m, 9H), 7.17-7.25(m, 2H), 7.30-7.55(m, 10H) , 7.66-7.80 (m, 2H), 7.90-8.00 (m, 2H), 8.10 (d, J=7.4Hz, 1H), 8.40-8.46 (m, 1H).
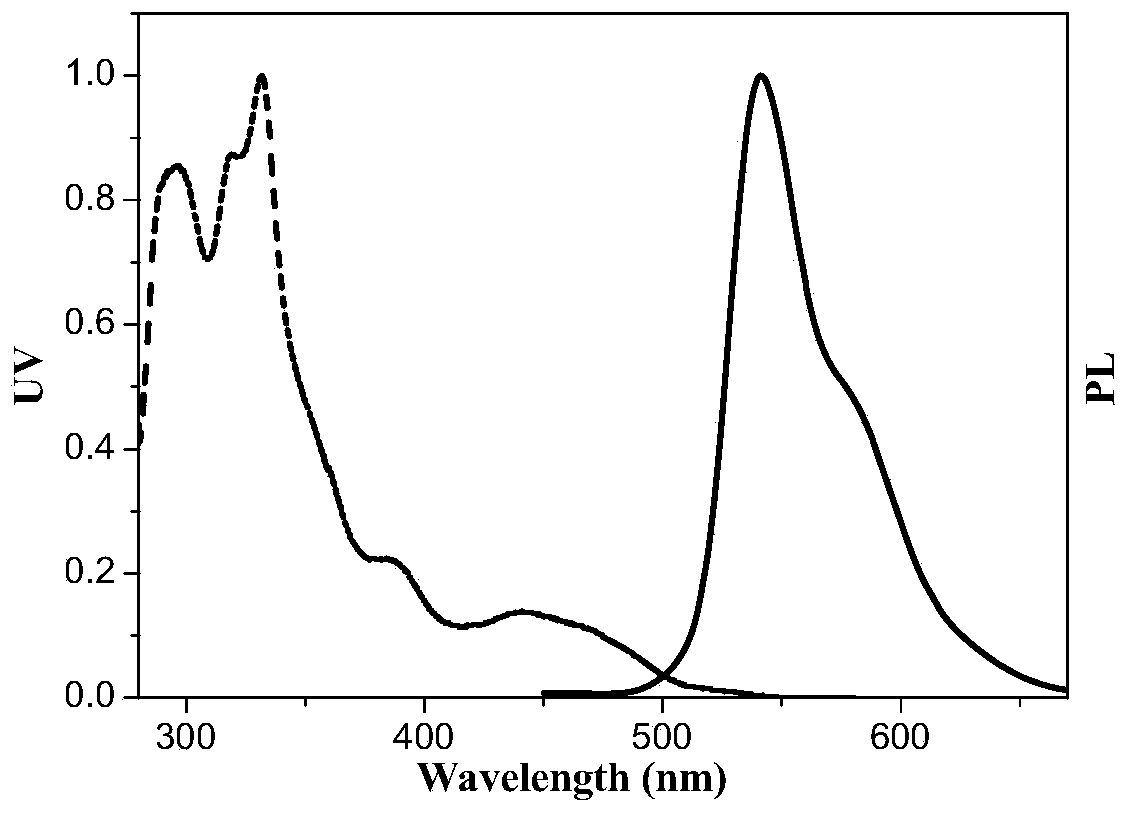
[0073]
Embodiment 3
[0075] A kind of preparation method of the discenyl-containing iridium complex of the present embodiment, 0.20g (0.1mmol) dichloro bridge intermediate, 0.054g (0.2mmol) 3-trifluoromethyl-5-(2-( 4-tert-butyl)pyridyl)pyrazole and 0.14g (1mmol) potassium carbonate were added into the locked tube, then 20mL of dichloromethane was added into the locked tube, and reacted at 60°C for 5h. After the reaction is complete, the product is separated by column chromatography. Get (TPS) 2 Ir(Bpz) orange-red solid 0.019g, yield 15%.(TPS) 2 The NMR spectrum of Ir(Bpz) is: 1 HNMR (400Hz, CDCl3) δ1.25(s, 9H), 5.22(s, 1H), 5.26(s, 1H), 5.92(d, J=7.6Hz, 1H), 5.98(d, J=7.5Hz, 1H), 6.07(d, J=8.5Hz, 1H), 6.45(d, J=4.8Hz, 2H), 6.70-6.84(m, 6H), 6.87-7.06(m, 9H), 7.16 (t, J=7.3Hz, 1H), 7.20(s, 1H), 7.28-7.39(m, 5H), 7.42-7.50(m, 4H), 7.51(s, 1H), 7.88(t, J=8.2Hz, 2H ). 19 F NMR (CDCl 3 , 367MHz) δ-59.81 (s, 3F).
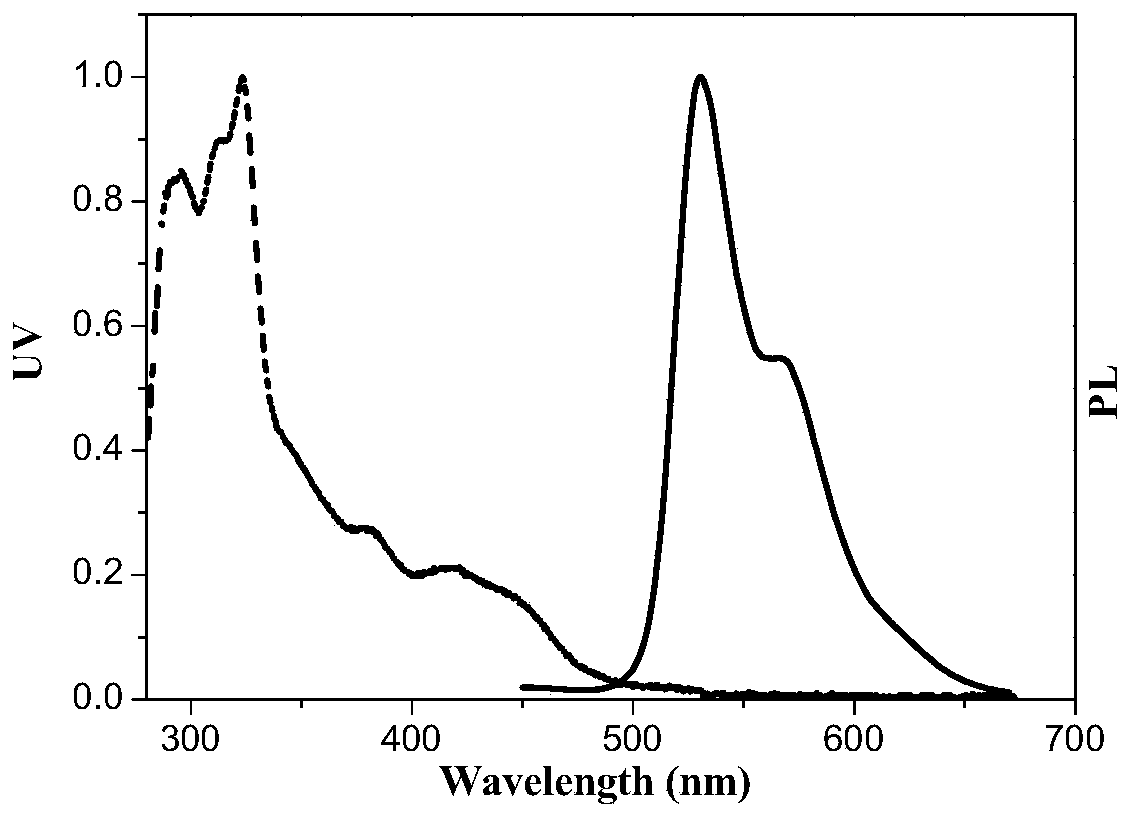
[0076]
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com