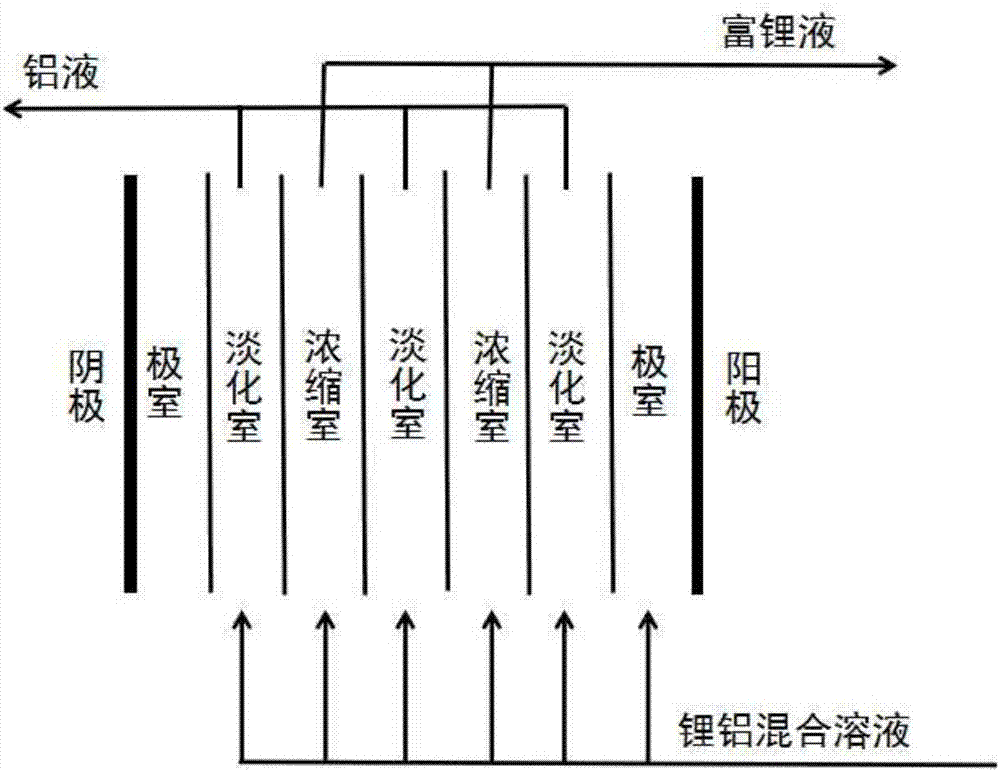
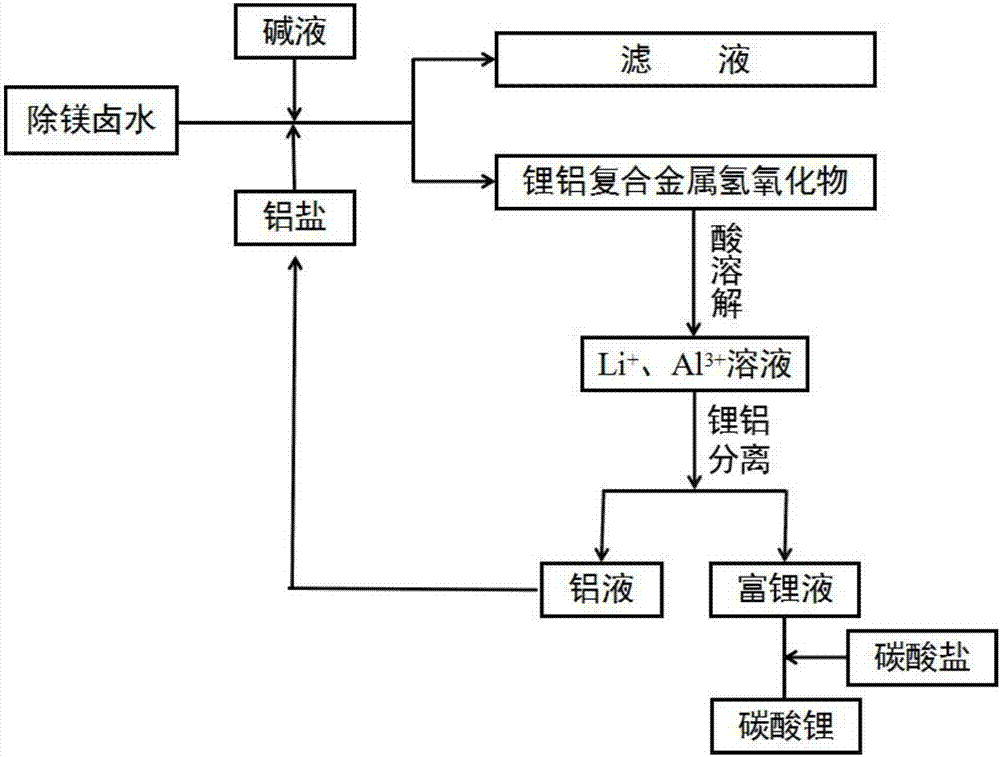
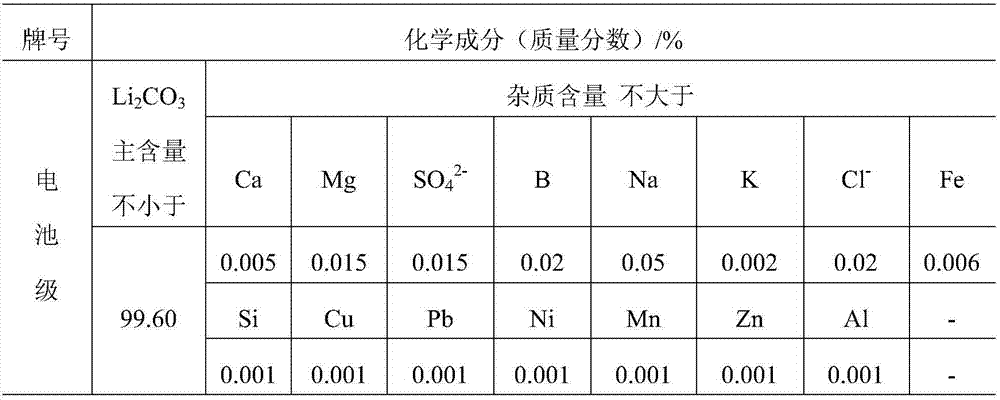
Method for extracting lithium from magnesium-removal bittern and preparing battery-grade lithium carbonate
A battery-grade, lithium carbonate technology, applied in the direction of lithium carbonate; Utilization rate and effect of small loss of lithium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A. Take 250mL of high-sodium brine after removing magnesium, in which the mass ratio of sodium to lithium is about 48, and add AlCl 3 ·6H 2 O 11.741g obtains mixed salt solution A;
[0042] B. Weigh 4.3767g of NaOH, NaOH 2 CO 3 5.1548g was dissolved in deionized water, and the 250mL volumetric flask was constant volume to obtain mixed alkali solution B;
[0043] C. Pour the mixed salt solution A and the mixed alkali solution B into the colloid mill at the same time, and rotate at a speed of 3000r / min for 3 minutes to form a magnesium-doped lithium-aluminum composite hydroxide crystal nucleus; transfer the crystal nucleus solution to the reactor, Crystallize with dynamic stirring at 80°C for 12 hours to grow;
[0044] D. Solid-liquid separation, filtration to obtain lithium-aluminum composite hydroxide filter cake, lithium-aluminum composite hydroxide filter cake was dried at 70 ° C for 12 hours to obtain a white solid product, its chemical formula is [LiAl 2 (OH) ...
Embodiment 2
[0051] A. Take 250mL of high-sodium brine after magnesium removal, wherein the mass ratio of sodium to lithium is about 48, add Al(NO 3 ) 3 9H 2 O18.243g obtains mixed salt solution A;
[0052] B. Weigh 4.3766g of NaOH, NaOH 2 CO 3 5.1548g was dissolved in deionized water, and the 250mL volumetric flask was constant volume to obtain mixed alkali solution B;
[0053] C. Pour the mixed salt solution A and the mixed alkali solution B into the colloid mill at the same time, and rotate at a speed of 3000r / min for 3 minutes to form a magnesium-doped lithium-aluminum composite hydroxide crystal nucleus; transfer the crystal nucleus solution to the reactor, Crystallize with dynamic stirring at 80°C for 12 hours to grow;
[0054] D. Solid-liquid separation, filtration to obtain lithium-aluminum composite hydroxide filter cake, lithium-aluminum composite hydroxide filter cake was dried at 70 ° C for 12 hours to obtain a white solid product, its chemical formula is [LiAl 2 (OH) 6...
Embodiment 3
[0061] A. Take 250mL of high-sodium brine after removing magnesium, in which the mass ratio of sodium to lithium is about 48, and add Al 2 (SO 4 ) 3 18H 2 O32.396g obtains mixed salt solution A;
[0062] B. Weigh 4.3769g of NaOH, NaOH 2 CO 3 5.1548g was dissolved in deionized water, and the 250mL volumetric flask was constant volume to obtain mixed alkali solution B;
[0063] C. Pour the mixed salt solution A and the mixed alkali solution B into the colloid mill at the same time, and rotate at a speed of 3000r / min for 3 minutes to form a magnesium-doped lithium-aluminum composite hydroxide crystal nucleus; transfer the crystal nucleus solution to the reactor, Crystallize with dynamic stirring at 80°C for 12 hours to grow;
[0064] D. Solid-liquid separation, filtration to obtain lithium-aluminum composite hydroxide filter cake, lithium-aluminum composite hydroxide filter cake was dried at 70 ° C for 12 hours to obtain a white solid product, its chemical formula is [LiAl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com