Recombinant pichia pastoris for heterologously and efficiently expressing rhizomucor miehei lipase and application of recombinant pichia pastoris
A technology of Rhizomucor miehei and lipase, which is applied in the biological field and can solve the problems of the influence of secretion amount on popularization and application, high activity of recombinant enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 Rhizomucor miehei cDNA
[0029] 1.1 Extraction of Rhizomucor militaris total RNA
[0030] (1) Take an appropriate amount of Rhizomucor miehei hyphae, absorb the water with filter paper, grind with liquid nitrogen, add 1ml Trizol reagent (Invitrogen), vibrate with an oscillator for 5 minutes, and let stand at room temperature for 1 minute;
[0031] (2) Add 0.2ml chloroform, shake for 15s, and let it stand for 2min;
[0032] (3) 4°C, 12000rpm, 15min;
[0033] (4) Aspirate the supernatant, add an equal volume of isopropanol, and precipitate at -20°C for 30 minutes;
[0034] (5) 4°C, 12000rpm, 15min;
[0035] (6) Pour off the supernatant, wash the precipitate with 1ml 75% ethanol, 7500rpm, 4°C, 5min;
[0036] (7) Repeat (6) step once;
[0037] (8) Pour off the supernatant and dry for 10 minutes;
[0038] (9) Add appropriate amount of DEPC water to dissolve to obtain total RNA;
[0039] 1.2 Preparation of the first strand of Rhizomucor ...
Embodiment 2
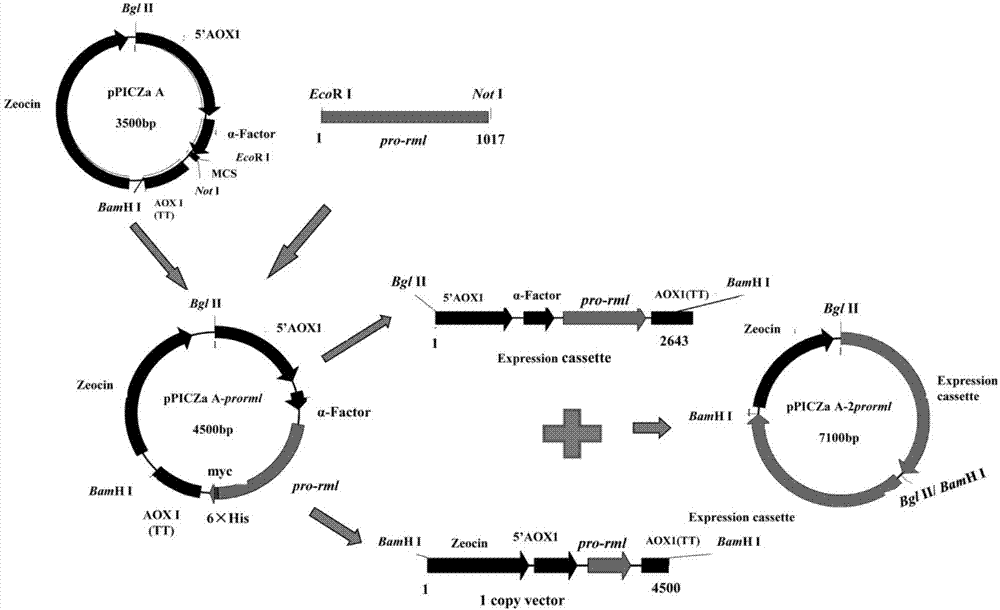
[0044] Example 2 Construction of 2 copies of the pPICZα A-2prorml plasmid
[0045] 2.1 Primer design
[0046] According to the sequence of the rml gene in GenBank (GenBank accession number is A02536.1), the following pair of primers were designed and synthesized:
[0047] FW(P1): 5'—CG GAATTC GTGCCAATCAAGAG—3' (SEQ ID NO.2)
[0048] REV (P2): 5'-TAG TCTAGA GTACAGAGGCCTGTG-3' (SEQ ID NO.3)
[0049] EcoR I and Not I restriction sites are designed at both ends of P1 and P2 respectively (see the italicized and underlined part in the above sequence)
[0050] 2.2 PCR amplification of Rhizomucor miehei lipase pro-rml containing leader peptide
[0051] Using P1 and P2 primers, Rhizomucor miehei (Boel E, Huge-Jensen B, Christensen M, Thim L, Fiil N:Rhizomucor miehei triglyceride lipase is synthesized as aprecursor.Lipids1988,23(7):701-706.) (Preserved in this room) cDNA is used as a template, and the PCR reaction system is:
[0052]
[0053] The reaction conditions are: 95°C ...
Embodiment 3
[0061] Example 3 Screening of Pichia pastoris recombinant strains with 4 copies of pro-rml gene
[0062] 3.1 Preparation of Pichia pastoris X-33 (purchased by Invitrogen) electroporation competent cells and their electroporation transformation
[0063] (1) Pick a fresh single colony and put it in 5ml YPD liquid medium, cultivate it at 30°C and 250rpm for 12-14 hours;
[0064] (2) Inoculate 0.1% of the inoculum into a 2L Erlenmeyer flask containing 500ml of YPD medium, cultivate at 30°C and 250rpm for 12-14 hours to make OD 600 =1.3-1.5;
[0065] (3) Centrifuge at 1500 rpm for 5 minutes at 4°C to collect the cells;
[0066] (4) Wash the cells twice with 500-250ml ice-cold sterile water;
[0067] (5) Wash the cells once with 20ml of ice-cold 1M sorbitol solution;
[0068] (6) Resuspend the cells with 1ml of ice-cold 1M sorbitol solution to a final volume of about 1.5ml, and dispense 80μl into small centrifuge tubes;
[0069] 3.2 Electric shock transformation of Pichia pasto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com