Method for co-producing sodium hexafluoroaluminate and biurea
A technology of sodium hexafluoroaluminate and biurea, which is applied in the chemical industry, can solve the problems of non-recycling and environmental pollution, and achieve the effect of no sewage discharge and favorable recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
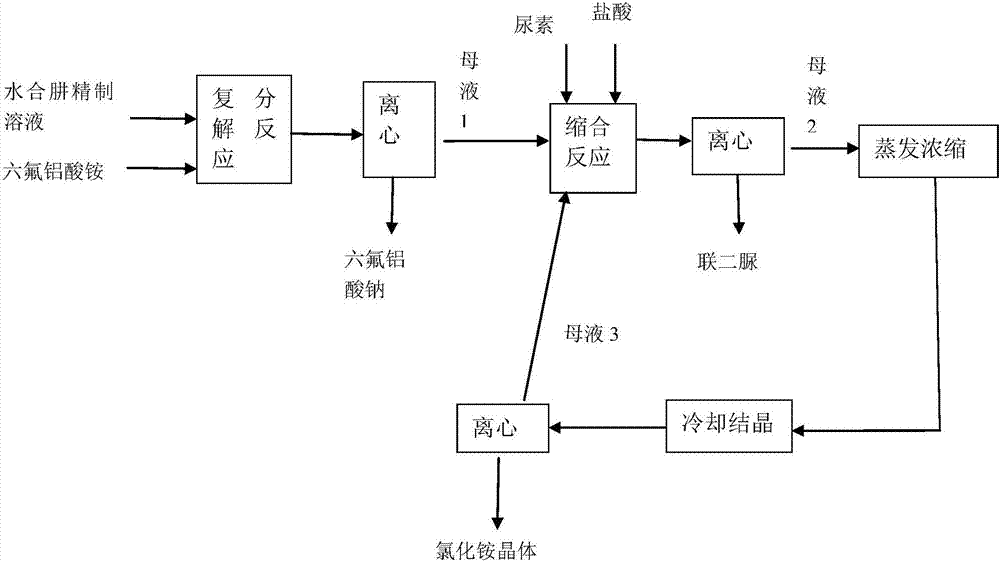
[0030] The block diagram of the technical process of the co-production of sodium hexafluoroaluminate and biurea in this embodiment is as follows figure 1 As shown, the specific steps are as follows:
[0031] 1. Put 300L sodium hypochlorite into 1m 3 In the reaction kettle, the contents of available chlorine and sodium hydroxide in sodium hypochlorite are 110g / L and 148g / L respectively, add urea solution containing 360g / L urea under stirring, with a volume of 82L, heat to 106°C with steam, and react to obtain hydrazine hydrate crude solution. Sampling and analysis showed that the crude solution contained hydrazine hydrate 52.2g / L, sodium carbonate 130g / L, urea 15g / L, sodium chloride 145g / L, and the rest was water. A volume of 367 L of crude solution containing hydrazine hydrate was obtained. Among them, the chemical reaction equation involved:
[0032] N 2 h 4 CO+NaClO+2NaOH=N 2 h 4 .H 2 O+Na 2 CO 3 +NaCl (1)
[0033] 2. Cool the crude solution of hydrazine hydrate ...
Embodiment 2
[0043] The block diagram of the technical process of the co-production of sodium hexafluoroaluminate and biurea in this embodiment is as follows figure 1 As shown, the specific steps are as follows:
[0044] 1. Put 200L sodium hypochlorite into 1m 3 In the reaction kettle, the contents of available chlorine and sodium hydroxide in sodium hypochlorite are 75g / L and 99g / L respectively, add urea solution containing 250g / L urea under stirring, with a volume of 55L, and heat it to 106°C with steam to obtain hydrazine hydrate crude solution. Sampling and analysis showed that the crude solution contained hydrazine hydrate 36.8g / L, sodium carbonate 87g / L, urea 10g / L, sodium chloride 97g / L, and the rest was water. A volume of 245 L of crude solution containing hydrazine hydrate was obtained. Among them, the chemical reaction equation involved:
[0045] N 2 h 4 CO+NaClO+2NaOH=N 2 h 4 .H 2 O+Na 2 CO 3 +NaCl (1)
[0046] 2. Cool the crude solution of hydrazine hydrate to -15°C...
Embodiment 3
[0056] The block diagram of the technical process of the co-production of sodium hexafluoroaluminate and biurea in this embodiment is as follows figure 1 As shown, the specific steps are as follows:
[0057] 1. Put 250L sodium hypochlorite into 1m 3 In the reaction kettle, the contents of available chlorine and sodium hydroxide in sodium hypochlorite are 95g / L and 125g / L respectively, add urea solution containing 315g / L urea under stirring, with a volume of 70L, heat to 106°C with steam, and react to obtain hydrazine hydrate crude solution. Sampling and analysis showed that the crude solution contained hydrazine hydrate 47g / L, sodium carbonate 110g / L, urea 13g / L, sodium chloride 122g / L, and the rest was water. A volume of 308 L of crude solution containing hydrazine hydrate was obtained. Among them, the chemical reaction equation involved:
[0058] N 2 h 4 CO+NaClO+2NaOH=N 2 h 4 .H 2 O+Na 2 CO 3 +NaCl (1)
[0059] 2. Cool the crude solution of hydrazine hydrate to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com