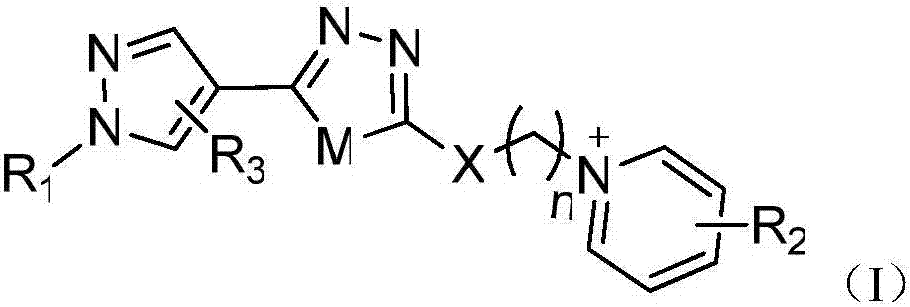
Pyrazolyl-oxa(thia)diazole compound containing pyridinium salt, and preparation method and application thereof
A technology of azole compounds and pyrazole oxadiazoles, which is applied in the field of pyrazole oxadiazole compounds and their preparation, and can solve problems such as inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
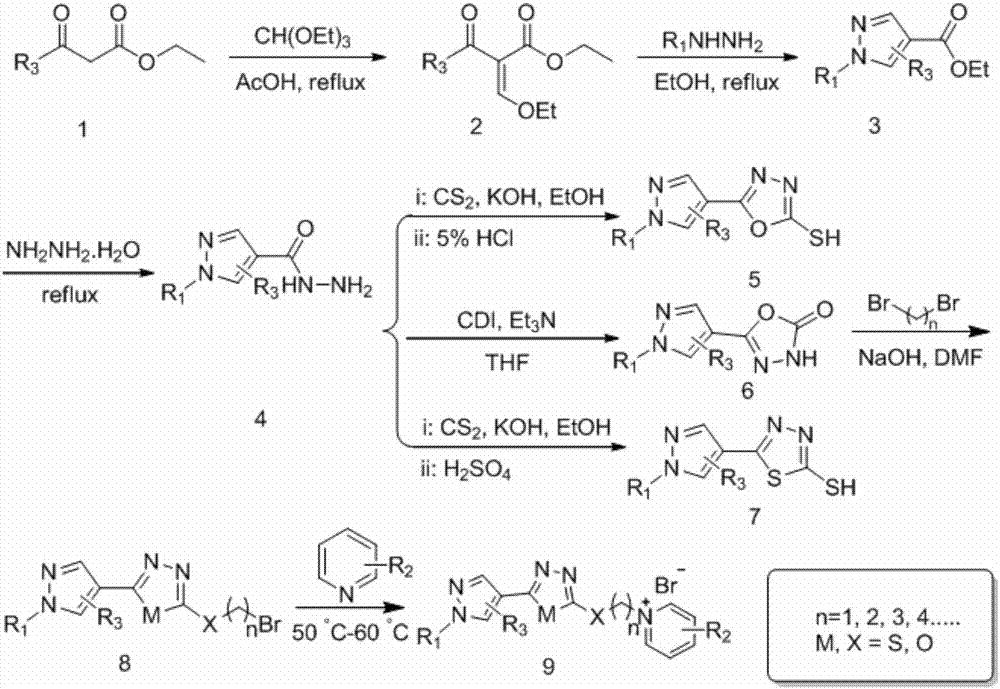
[0023] Example 1 of the present invention: target compound 10-(2-thioether-5-(1phenyl-2-trifluoromethyl)pyrazolyl)-1,3,4-oxadiazole-decyl-1 - Preparation of pyridinium bromide salt
[0024] Dissolve 0.5g 2-(10-bromodecyl)thioether-5-(1phenyl-2-trifluoromethyl)pyrazolyl-1,3,4-oxadiazole in 5mL pyridine, 50℃ The reaction was carried out for 6h, desolventized, and purified by column chromatography to obtain 0.53g of yellow liquid with a yield of 92.0%.
[0025] Refer to Example 1 for the synthesis of thioether and oxyether target compounds.
[0026] The structures, hydrogen NMR, carbon and fluorine spectra and mass spectrometry data of some of the synthesized pyrazole dioxa (thi) diazole compounds containing pyridine salts are shown in Table 1, and the physicochemical properties are shown in Table 2.
[0027] H NMR and C NMR data of some compounds in Table 1
[0028]
[0029]
[0030]
[0031]
[0032]
[0033] Table 2 Physicochemical properties of some target c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com