Method for synthesizing N-Boc-L-propargyl glycine
A technology of propargyl glycine and a synthesis method, which is applied in the synthesis field of N-Boc-L-propargyl glycine, can solve the problems of strict control of process parameters, large loss of raw materials, inconvenient operation, etc., and achieves reduction of purification and treatment process, improve ee value, reduce the effect of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
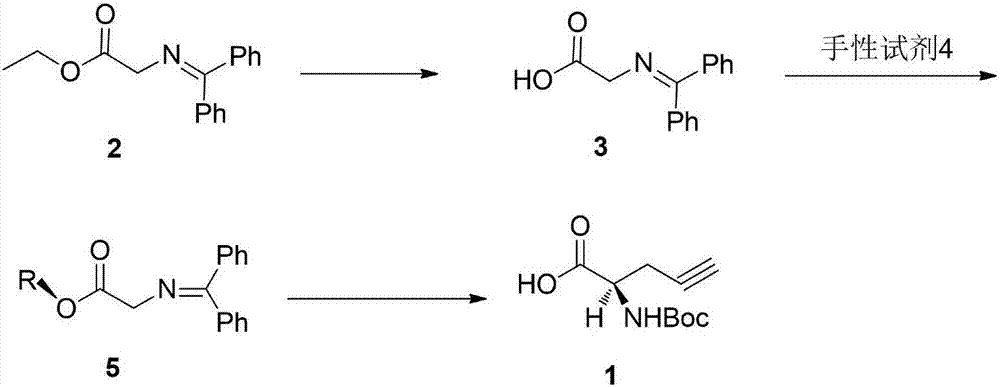
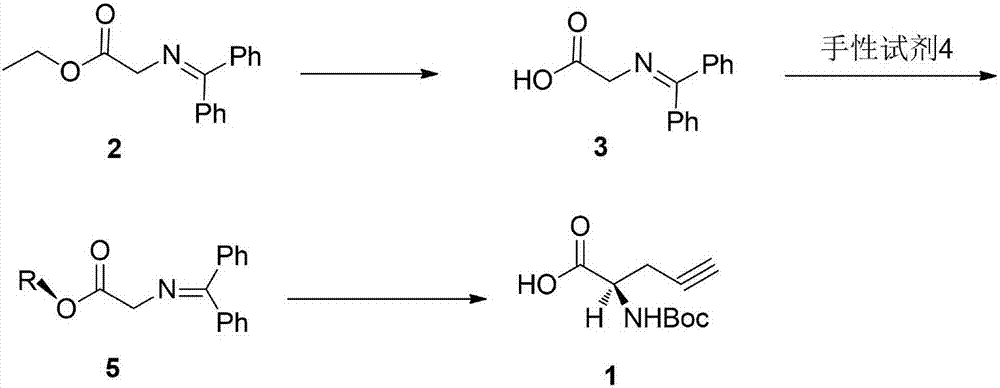
[0041] Preparation of compound 3
[0042] Add 26.7g (0.1mol) of compound 2 to 300ml of 1mol / L sodium hydroxide aqueous solution (0.3mol), stir at 25°C until it is completely dissolved, that is, the reaction is over, adjust the pH to 7 with 1mol / L hydrochloric acid, a large amount of white The solid precipitated out and was suction-filtered. The filter cake was washed with 50 ml of water and dried to obtain 22.7 g of compound 3 with a yield of 95% and a purity of 98.0%.
[0043] Preparation of compound 5
[0044] Add 30g (0.125mol) of compound 3, 29g (-)-1R, 2S, 5R-8-phenylmenthol (0.125mol), and 300ml of dichloromethane into a 500ml reaction flask in turn, start stirring, and wait for the solid to dissolve After that, add 1.5g (0.0125mol) of 4-dimethylaminopyridine, and react at 25°C for 16h. After the reaction is completed, the reaction solution is washed three times with saturated brine, concentrated to dry light yellow solid, and washed with 100ml of ethanol / water (3:1 ) ...
Embodiment 2
[0048] Preparation of compound 3
[0049] Add 26.7g (0.1mol) of compound 2 to 500ml of 1mol / L potassium hydroxide aqueous solution (0.5mol), stir at 25°C until it is completely dissolved, and the reaction is completed, adjust the pH to 7 with 1mol / L hydrochloric acid, a large amount of white solid Precipitate, filter with suction, wash the filter cake with 100ml of water, and dry to obtain 21.5g of compound 3, with a yield of 90% and a purity of 98.1%.
[0050] Preparation of compound 5
[0051] Add 30g (0.125mol) of compound 3, 32.2g (-)-1R, 2S, 5R-8-naphthyl menthol (0.139mol), and 300ml of dichloromethane into a 500ml reaction flask in turn, start stirring, and wait until the solid After dissolving, add 1.5g (0.0125mol) of 4-dimethylaminopyridine, and react at 25°C for 16h. After the reaction is completed, the reaction solution is washed three times with saturated brine, concentrated to dryness as a light yellow solid, and washed with 100ml of ethanol / water (3: 1) Heat to...
Embodiment 3
[0055] Preparation of compound 3
[0056] Add 26.7g (0.1mol) of compound 2 to 200ml of 1mol / L sodium hydroxide aqueous solution (0.2mol), stir at 25°C until it is completely dissolved, that is, the reaction is over, adjust the pH to 7 with 1mol / L hydrochloric acid, and a large amount of white The solid was precipitated, filtered with suction, the filter cake was washed with 50 ml of water, and dried to obtain 22.6 g of compound 3 with a yield of 94.6% and a purity of 98.0%.
[0057] Preparation of compound 5
[0058] Add 30g (0.125mol) of compound 3, (-)-1R, 2S, 5R-8-(4-methoxymethylbenzene) menthol (0.125mol), and 300ml of dichloromethane into a 500ml reaction flask in sequence , start stirring, after the solid dissolves, add 1.5g (0.0125mol) 4-dimethylaminopyridine, react at 25°C for 16h, the reaction is completed, the reaction solution is washed three times with saturated saline, concentrated to dry light yellow solid, and used 100ml Ethanol / water (5:1) was heated to diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com