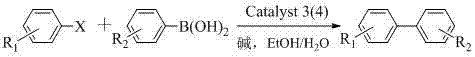Self-loading diphosphine-palladium catalyst and preparation method and application thereof
A self-supporting bisphosphine and palladium catalyst technology, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of metal catalyst product pollution, low catalytic activity, etc., and achieve excellent catalytic effect, high heat Stability, simple and feasible reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
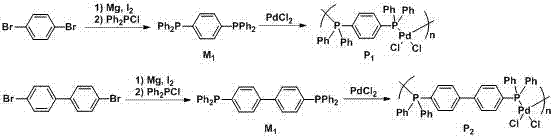
[0028] Embodiment 1-8: the preparation method of bisphosphine-palladium catalyst.
[0029]
Embodiment 1
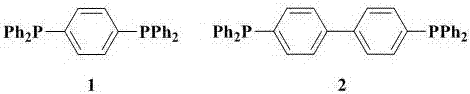
[0031] Magnesium chips (144 mg, 6 mmol), 1 capsule (about 35 mg, the same below) iodine and 1,4-dibromobenzene (468 mg, 2 mmol) dissolved in dry N,N'-dimethylformaldehyde Amide (6 mL), reacted at 40 °C for 6 h, added diphenylphosphorous chloride (880 mg, 4 mmol), stirred at room temperature for 8 h, washed with ammonium chloride aqueous solution after the reaction, extracted with ethyl acetate, dried, and passed through the column Chromatography (petroleum ether as developer) gave 1 (268 mg, 60%) as a white solid. M.p. 166-167 °C; IR (KBr): υ 3053, 1478, 1433, 1092, 821, 741, 694, 552, 513, 483 cm -1 ; 1 H-NMR (600 MHz, CDCl 3 , 25 ℃, TMS): δ = 7.22-7.24 (m, 4H), 7.31-7.33 (m, 20H); 13 C-NMR (150 MHz, CDCl 3 , 25℃, TMS): δ = 128.49, 128.82, 133.34, 133.80, 136.71, 137.93; 31P-NMR (240MHz,CDCl 3 , 25 ℃, TMS): δ = -6.64.
Embodiment 2
[0033] Dissolve magnesium chips (288 mg, 12 mmol), 1 grain of iodine and 1,4-dibromobenzene (624 mg, 2 mmol) in dry tetrahydrofuran (6 mL), react at 60 °C for 8 h, add diphenylphosphine Chlorine (880 mg, 4 mmol), stirred at room temperature for 8 h, washed with ammonium chloride aqueous solution after the reaction, extracted with ethyl acetate, dried, and obtained white solid 1 (335 mg, 75 %). M.p. 166-167 °C; IR (KBr): υ 3053, 1478, 1433, 1092, 821, 741, 694, 552,513, 483 cm -1 ; 1 H-NMR (600 MHz, CDCl 3 , 25 ℃, TMS): δ = 7.22-7.24 (m, 4 H),7.31-7.33 (m, 20 H); 13 C-NMR (150 MHz, CDCl 3 , 25 ℃, TMS): δ= 128.49, 128.82, 133.34, 133.80, 136.71, 137.93; 31 P-NMR (240 MHz, CDCl 3 , 25 ℃, TMS): δ=-6.64.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com