Preparation method of crosslinked nanomedicine based on active reaction one-step method
A nano-drug, reactive technology, applied in the direction of drug combination, pharmaceutical formula, non-active ingredient medical preparations, etc., can solve the problems of complex multi-step preparation of chemically bonded nano-drugs, non-crosslinking of chemically-bonded nano-drugs, etc. Achieve the effect of avoiding non-crosslinking, increasing maximum tolerance, and reducing drug leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] cRGD-PS-DM1 cross-linked nanomedicine was directly formed by co-polymer PEG-P(TMC-DTC) and cRGD-PEG-P(TMC-DTC) (w / w 80 / 20) encapsulating DM1 while self-assembling, The invention creatively realizes sulfur-sulfur-sulfhydryl exchange reaction and sulfur-sulfur cross-linking simultaneously.
[0043] The overall reaction idea of this embodiment is:
[0044]
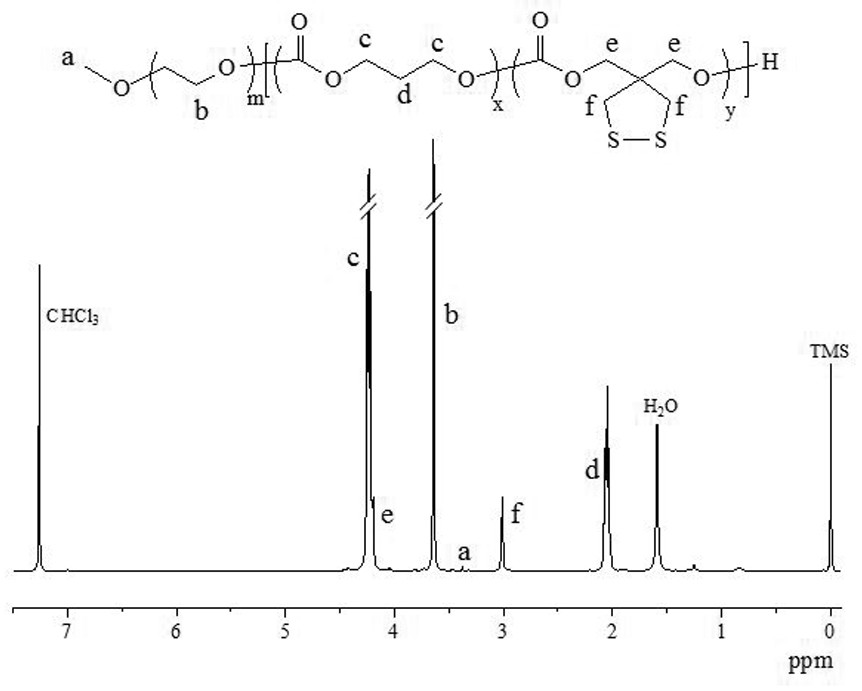
[0045] Under nitrogen protection, NHS-PEG-OH ( M n = 7.5 kg / mol, 75 mg, 10 μmol), TMC (200 mg, 1.6 mmol) and DTC (50 mg, 0.26 mmol) were dissolved in 1.1 mL of anhydrous dichloromethane, followed by adding diphenyl phosphate (DPP, 25 mg, 100 μmol) in a closed reactor at 30°C for 72 h, terminated, precipitated twice in glacial ether, filtered with suction and dried in vacuo overnight to obtain NHS-PEG-P(TMC-DTC). Yield: 84.6%. 1 H NMR (600 MHz, CDCl 3 ): PEG: δ 3.64; TMC: δ 2.06, 4.24; DTC: δ 3.02, 4.19; NHS: δ 2.52. M n ( 1 HNMR) =31.8 kg / mol. M n (GPC) = 34.8 kg / mol, M w / M n = 1.14; then the o...
Embodiment 2
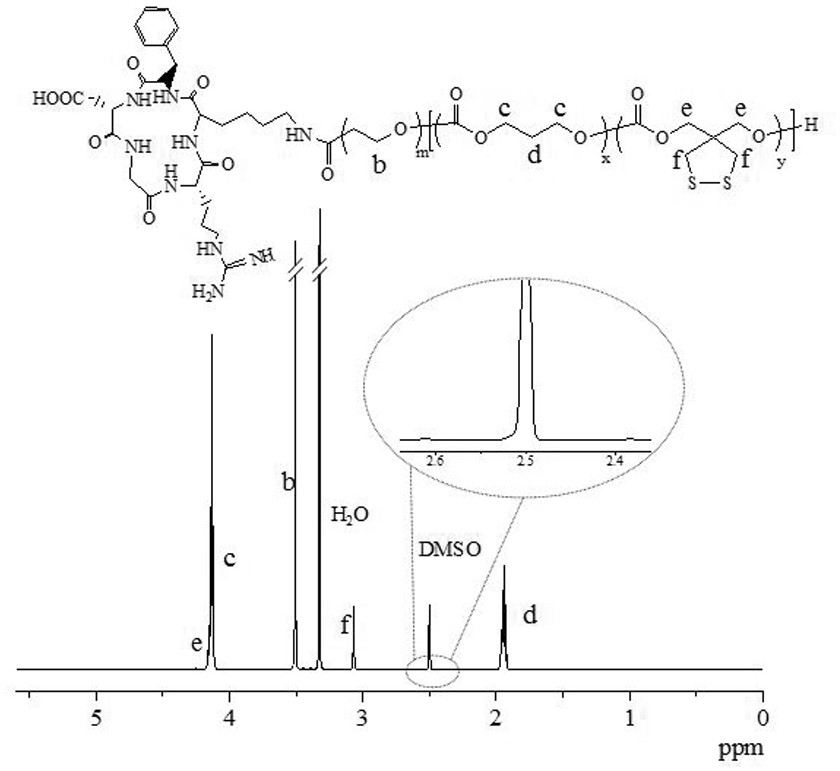
[0065] According to Example 1, the polymers PEG-P (TMC-DTC) and MAL-PEG-P (TMC-DTC) were prepared, and the targeting molecule Anti-CD19-PEG of the anti-CD19 antibody was prepared from the latter -P(TMC-DTC), the chemical structure is as follows:
[0066]
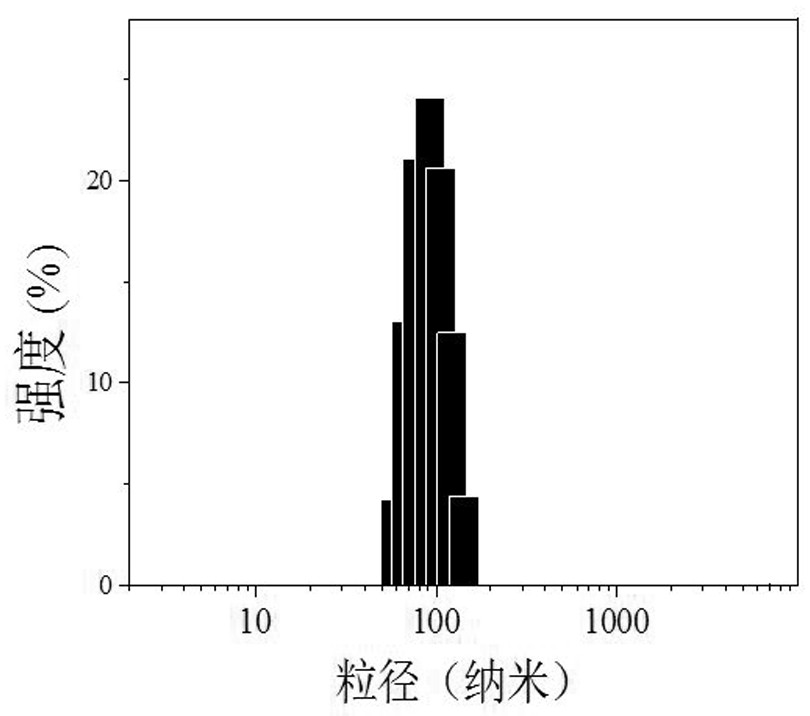
[0067] Further preparation of cross-linked nano-drugs and targeted cross-linked nano-drugs, which are vesicle structures with a particle size of about 90-100 nm; loaded with maytansine DM1, the drug loading DLC can reach 10-15wt.%; MTD research results show The MTD values of cross-linked vesicle nanomedicine and targeted cross-linked vesicle nanomedicine are nearly 4 times higher than that of free DM1; the tumor inhibition rate (TIR ) respectively exceeded 60% and 90%; the median survival of mice exceeded 35 days and 60 days (PBS was 25 days), and the toxic and side effects on the main organs of mice were very small.
Embodiment 3
[0069] According to Example 1, TMC was replaced with LA to prepare polymers PEG-P (LA-DTC) and NHS-PEG-P (LA-DTC), and the targeting molecule was prepared from the latter to target polymerization of EGFR targeting polypeptide GE11 Material GE11-PEG-P (LA-DTC), the chemical structure is as follows:
[0070]
[0071] Further preparation of cross-linked nano-drugs and targeted cross-linked nano-drugs, which are vesicle structures with a particle size of about 50-70 nm; loaded with the drug 6-mercaptopurine (6MP) with its own mercapto group, and the drug loading DLC is 5- 10 wt.%; MTD results showed that the MTD value of cross-linked vesicle nano-drugs and targeted cross-linked vesicle nano-drugs was 6 times that of free 6MP; The tumor inhibition rate (TIR) of triple-negative breast cancer MDA-MB-231 subcutaneous tumors exceeded 60% and 85% respectively; the median survival of mice exceeded 47 days and 58 days (PBS was 30 days), and the main effect on mice Visceral almost ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com