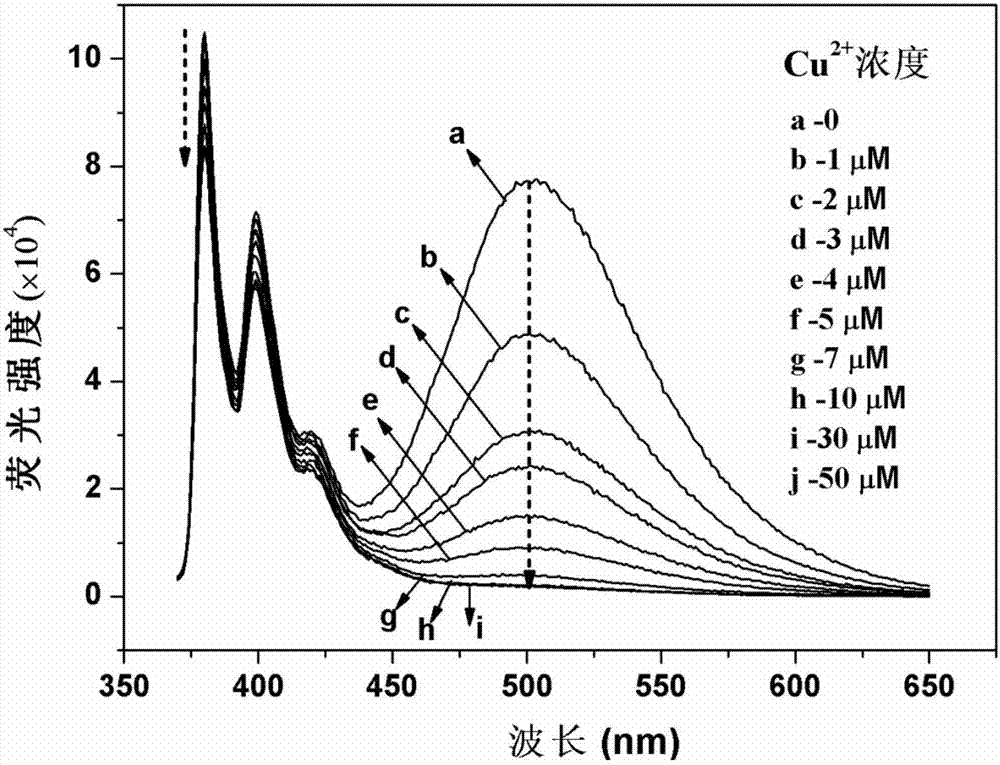
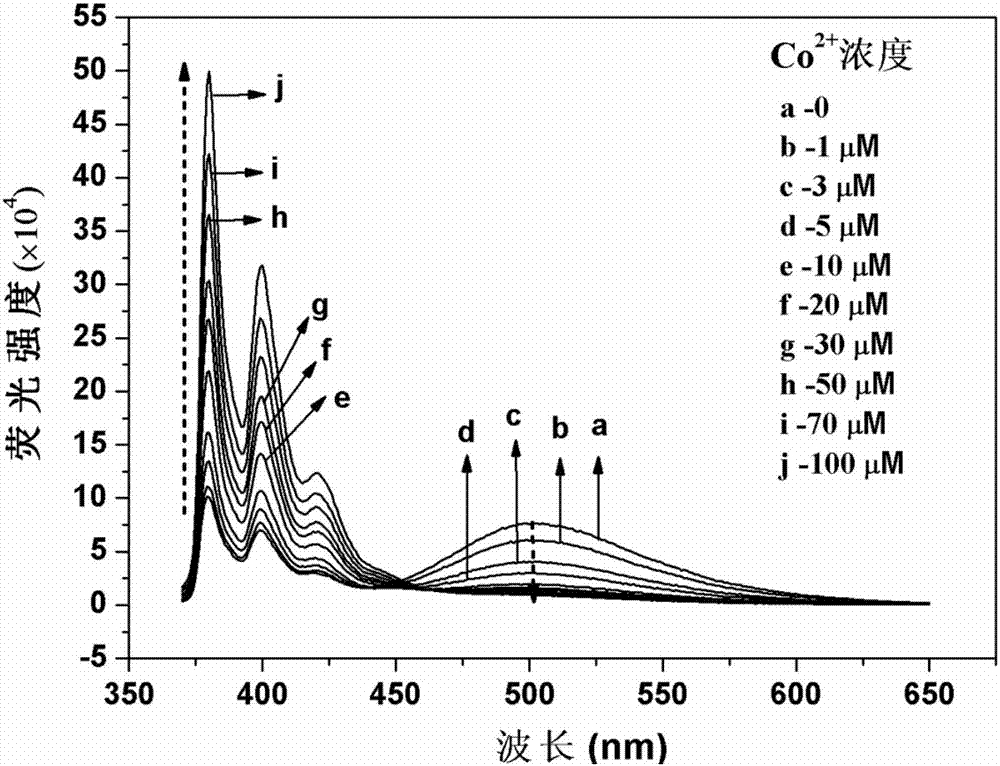
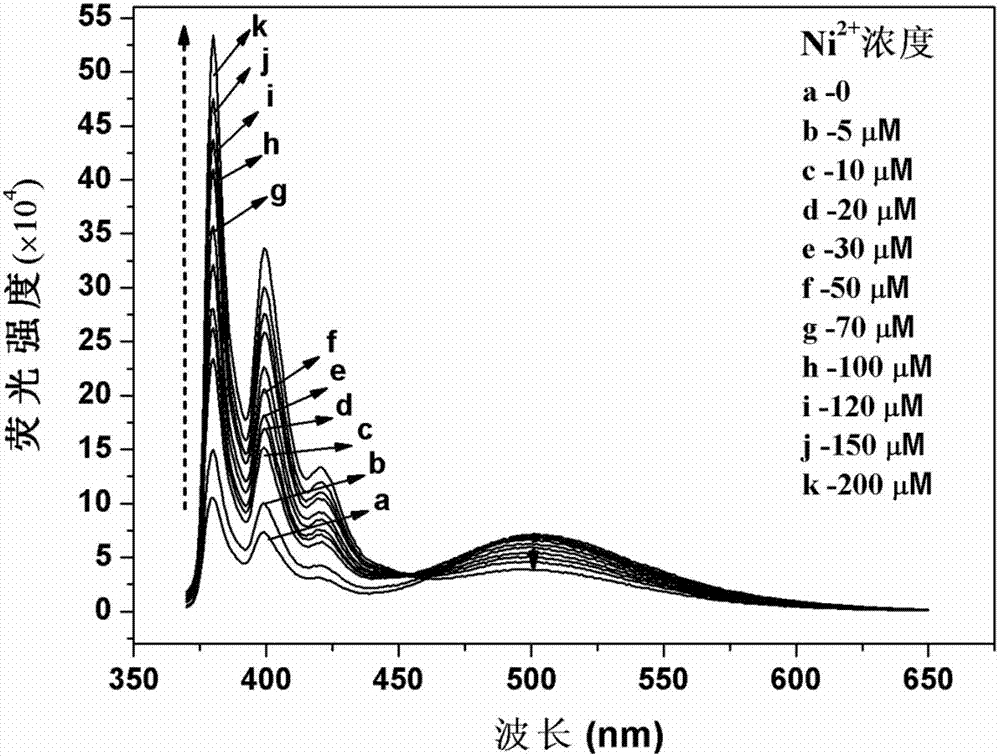
Di(2-methylpyridine)amine modified pyrene derivative fluorescence probe, and synthetic method and applications thereof
A picoline and fluorescent probe technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of large sample consumption, cumbersome data acquisition and processing, etc., and achieve good biocompatibility and production High rate and good discrimination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1. Synthesis of pyrenesulfonyl derivatives
[0054] Under the conditions of nitrogen protection and ice-bath stirring at a flow rate of 0.6mL / s, 1.48g (9.97mmol) of 1,8-diamino-3,6-dioxoctane was added to a three-necked flask containing 60mL of chloroform , and then 0.3g (0.997mmol) pyrenesulfonyl chloride was dissolved in 50mL chloroform and then added dropwise to the three-necked flask at a rate of 6 s / drop, after the dropwise addition, stirred at room temperature for 2 hours, and the reaction solution was washed with saturated saline for 5 ~8 times, then dried overnight with anhydrous sodium sulfate, washed with 1mol / L hydrochloric acid, and then neutralized with 3mol / L aqueous sodium hydroxide solution to make the pH value of the solution 7, then extracted with chloroform, collected organic layer, spin-dried to obtain pyrenesulfonyl derivatives (Py-EOA), and its reaction equation is as follows:
[0055]
[0056] 2. Synthesis of two (2-picoline) amine derivatives...
Embodiment 2
[0065] The pyrene derivative fluorescent probe modified by bis(2-picoline)amine with the following structural formula was synthesized:
[0066]
[0067] In step 1 of the synthesis of pyrenesulfonyl derivatives in Example 1, the 1,8-diamino-3,6-dioxoctane used was mixed with an equimolar amount of 3,6,9-trioxaundecane - 1,11-diamine replacement, the other steps are the same as in Example 1 to obtain a bis(2-picoline)amine-modified pyrene derivative fluorescent probe.
Embodiment 3
[0069] The pyrene derivative fluorescent probe modified by bis(2-picoline)amine with the following structural formula was synthesized:
[0070]
[0071] In step 1 of the synthesis of pyrenesulfonyl derivatives in Example 1, the 1,8-diamino-3,6-dioxoctane used was mixed with an equimolar amount of 3,6,9,12-tetraoxadecane Hexane-1,16-diamine was replaced, and the other steps were the same as in Example 1 to obtain a bis(2-picoline)amine-modified pyrene derivative fluorescent probe.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap