Au-TiO(2-x) catalyst and application thereof
A technology of au-tio2-x and catalyst, which is applied in the direction of physical/chemical process catalyst, catalyst carrier, metal/metal oxide/metal hydroxide catalyst, etc. It can solve the problem of poor TiO2 carrier effect and low catalyst WGS activity , active metal interaction and other problems, to achieve the effect of improving thermal current transmission efficiency, reducing ohmic energy barrier, and promoting reduction reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
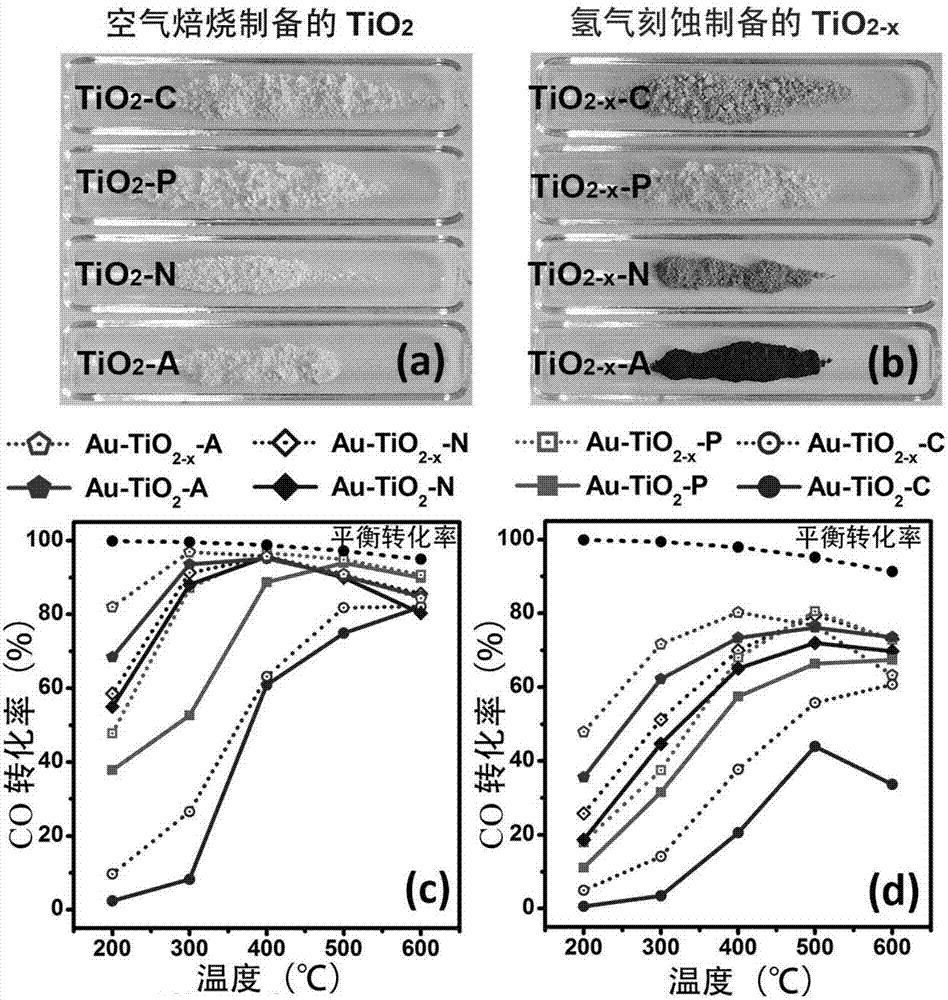
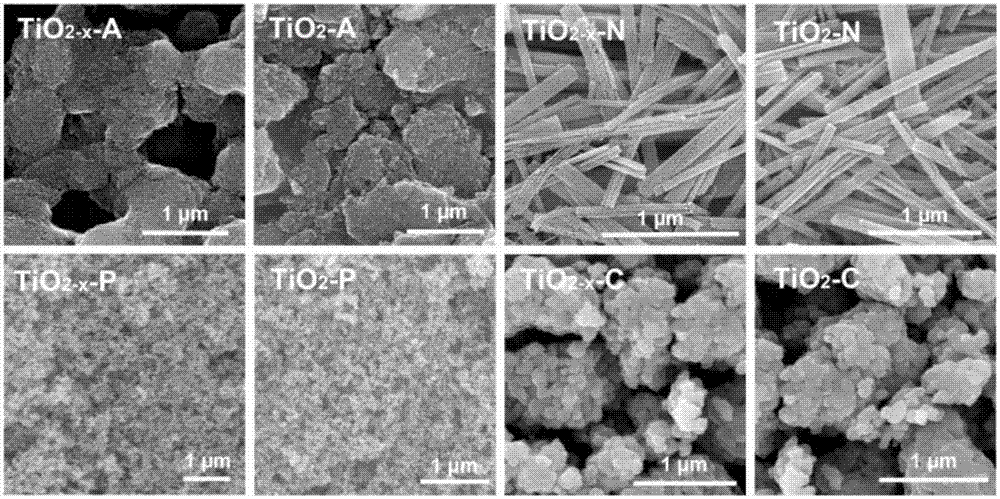
[0064] Example 1Au-TiO 2 -A and Au-TiO 2-x -Preparation of catalyst
[0065] 1. TiO 2 -A and TiO 2-x - Preparation of A carrier
[0066] (1) Add 4.65g of glacial acetic acid and 120mL of distilled water into a 500mL round bottom flask, then slowly add 20mL of tetra-n-butyl titanate, and stir vigorously at 80°C for 8 hours;
[0067] (2) Transfer the mixture in the round bottom flask to a 120mL polytetrafluoroethylene-lined autoclave, and keep it in an electric oven at 180°C for 24 hours to obtain a precipitate;
[0068] (3) The precipitate was washed with deionized water until the pH of the supernatant was neutral, and dried at 100 °C for 12 hours to obtain solid TiO 2 ;
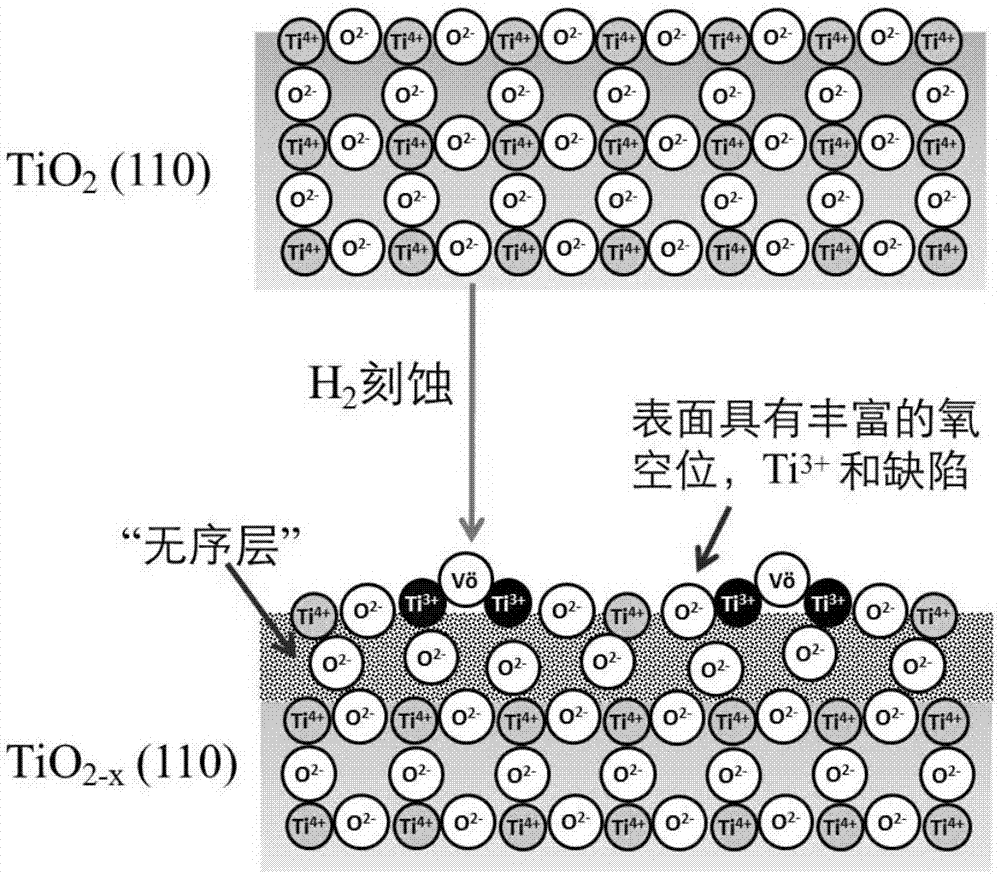
[0069] (4) the solid TiO 2 Calcined at 550 °C for 4 hours in air atmosphere to obtain white anatase TiO 2 (TiO 2 -A); or solid TiO 2 in H 2 Calcined at 550°C for 4 hours in the atmosphere to obtain black anatase TiO 2 (TiO 2-x -A).
[0070] 2. Au-TiO 2 -A and Au-TiO 2-x -Preparation of catalys...
Embodiment 2A
[0073] Example 2Au-TiO 2 -N and Au-TiO 2-x Preparation of -N catalyst
[0074] 1. TiO 2 -N and TiO 2-x Preparation of -N carrier
[0075] (1) Add 6g of tetra-n-butyl titanate into 18mL of distilled water, and continue to stir for 30min after ultrasonication for 30min; add another 17g of NaOH into 21mL of distilled water, and stir evenly;
[0076] (2) After mixing the two, ultrasonicate for 15 minutes, and continue to stir until milky, then add it to a 50mL polytetrafluoroethylene-lined autoclave, and place it in an electric oven at 200°C for 48 hours to obtain sodium titanate (Na 2 Ti 3 o 7 ) nanoribbons;
[0077] (3) After the reaction, wash the sodium titanate (Na titanate) with distilled water 2 Ti 3 o 7 ) nanoribbons, at 0.1M HNO 3 acidified overnight and dried at room temperature to give the acidified titanate (H 2 Ti 3 o 7 );
[0078] (4) The acidified titanate (H 2 Ti 3 o 7 ) was calcined at 550°C for 4 hours in an air atmosphere to obtain white TiO ...
Embodiment 3A
[0082] Example 3Au-TiO 2 -C and Au-TiO 2-x Preparation of -C catalyst
[0083] Commercial TiO 2 (Shanghai Chemical Reagent Co., Ltd.) calcined in air at 550°C for 4 hours to obtain white TiO 2 -C; alternatively, commercial TiO 2 (Shanghai Chemical Reagent Co., Ltd.) at 550°C in H 2 Calcined in the atmosphere for 4 hours to obtain gray TiO 2-x -C.
[0084] Au-TiO 2-x The preparation method of the -C catalyst is the same as that of the catalyst in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystal size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com