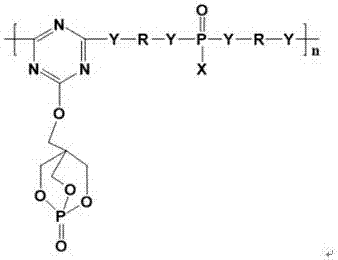
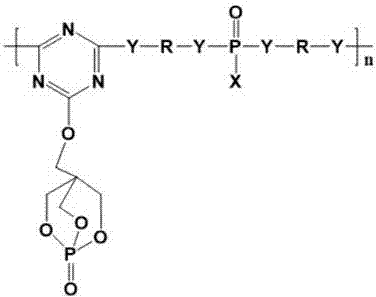
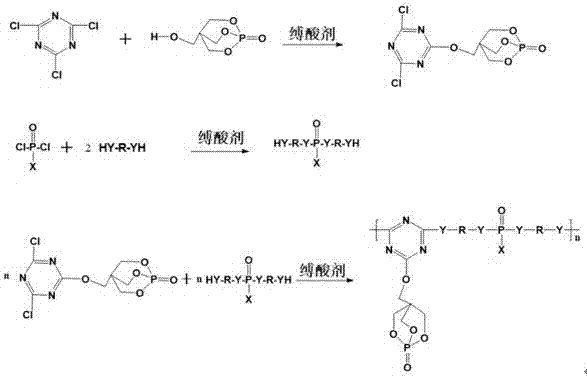
Trine expansion type flame retardant and synthetic method thereof
An intumescent flame retardant, a trinity technology, applied in the field of "trinity" intumescent flame retardant and its synthesis, can solve the problems of high moisture absorption, insufficient proportion, and many hydroxyl groups in the flame retardant, and achieve good results. Excellent charcoal properties, thermal stability, and flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] step one
[0038] In a 1000ml three-necked flask equipped with a reflux condenser, a stirrer, and a constant pressure dropping funnel, add 92.25g (0.5mol) of cyanuric chloride, then add 200ml of acetonitrile, place the three-necked flask in an oil bath, and fully Stir to disperse cyanuric chloride evenly, and drop 90.0g (ie 0.5mol) of 1-oxyphospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2. 2] Octane (PEPA), 50.6g (0.5mol) of triethylamine was added dropwise with a constant pressure funnel, the pH was controlled between 5-7, the reaction temperature was controlled at 35°C, and the reaction time was 3 hours to obtain three A monobasic substitute for polycyanogen chloride (CNC-PEPA).
[0039] step two
[0040] Add a mixture of ethylenediamine 60.10 g (1mol), chloroform (350mL) and triethylamine 101.19g (1mol) into another 1000mL three-neck flask, cool to 0°C in an ice bath or freezing reactor, and slowly 97.49 g (0.5 mol) of phenylphosphoryl dichloride was added dropwise ...
Embodiment 2
[0044] step one
[0045] In a 500ml three-necked flask equipped with a reflux condenser, a stirrer, and a constant pressure dropping funnel, add 46.13g (0.25mol) of cyanuric chloride, then add 200ml of acetone, place the three-necked flask in an oil bath, and fully Stir to disperse cyanuric chloride evenly, add 45.0g (0.25mol) of PEPA dropwise to the three-necked bottle, add 25.3g (0.25mol) of triethylamine dropwise with a constant pressure funnel, and control the pH between 5-7 During this period, the reaction temperature was controlled at 30°C and the reaction time was 3 hours to obtain the monosubstituted cyanuric chloride (CNC-PEPA).
[0046] step two
[0047] Add 30.05g (0.5mol) of ethylenediamine and 50.60g (0.5mol) of triethylamine mixture and solvent dioxane (250mL) into another 500mL three-neck flask, and cool to 10°C in a refrigerated reactor. Slowly add 62.5 g (0.25 mol) of anilinophosphoryl dichloride dropwise and react for 4 hours to obtain a mixture. The solven...
Embodiment 3
[0051] step one
[0052] In a 1000ml three-necked flask equipped with a reflux condenser, a stirrer, and a constant pressure dropping funnel, add 184.5g (1mol) of cyanuric chloride, then add 500ml of acetone, place the three-necked flask in an oil bath, and stir thoroughly , to make the cyanuric chloride disperse evenly, add 180.0g (1mol) PEPA dropwise to the there-necked bottle, add 101.19g (1mol) triethylamine dropwise with a constant pressure funnel, control the pH between 5-7, and the reaction temperature The temperature is controlled at 30°C, and the reaction time is 3 hours to obtain the monobasic substituted product of cyanuric chloride (CNC-PEPA).
[0053] step two
[0054] Add 124.18 g (2mol) of ethylene glycol, chloroform (400mL) and 202.38g (2mol) of triethylamine mixture into a 1000mL three-necked flask, cool to -10°C in an ice-bath reactor, and slowly add 211.0 g (1mol) of phenoxyphosphoryl dichloride was reacted for 5 hours to obtain a mixture, and the solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com