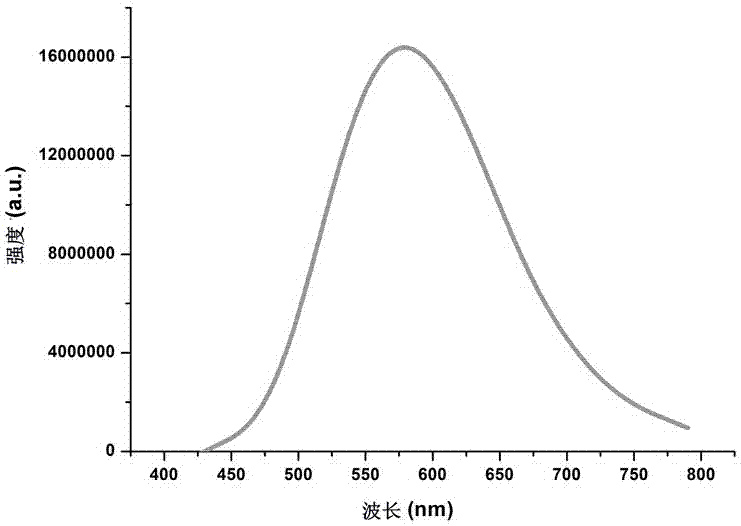
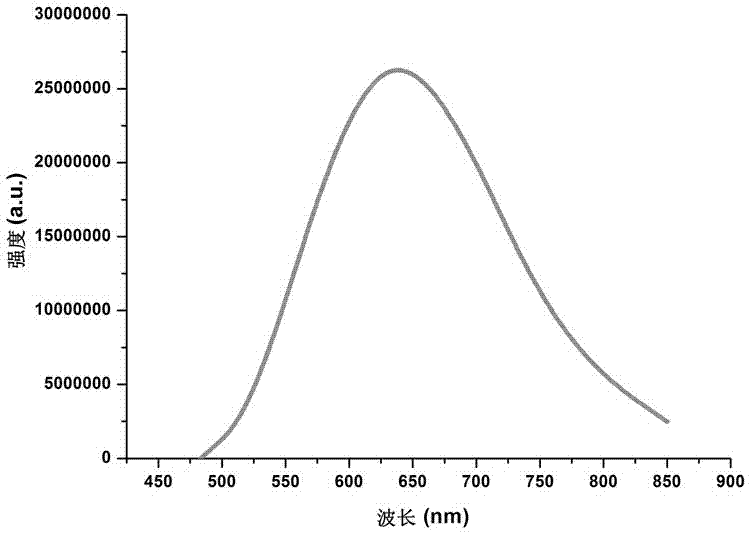
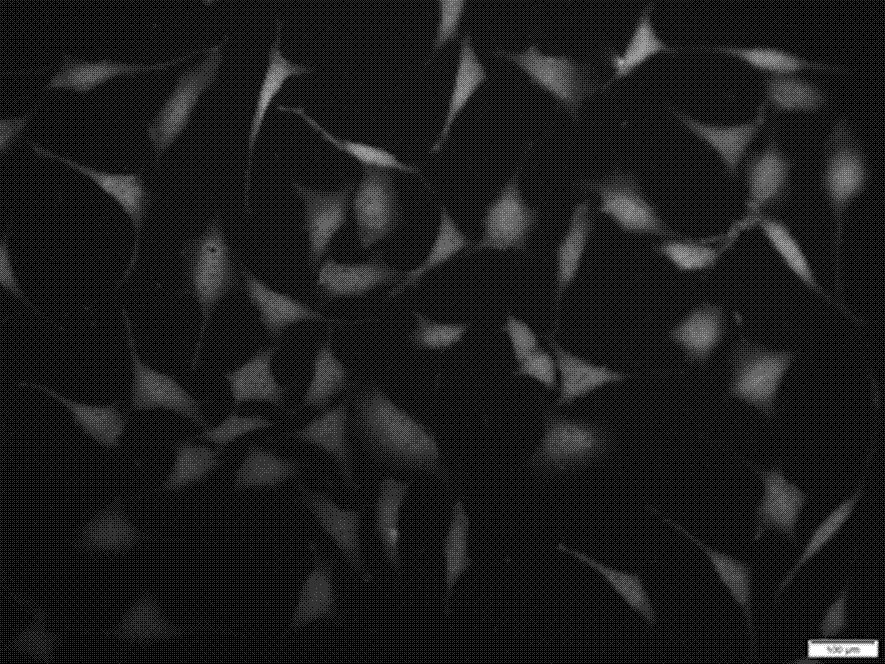
Water-soluble fluorescent dye for cell fluorescent developing
A technology of compound and general formula, which is applied in the field of synthesis of water-soluble fluorescent dye compounds, can solve the problems of unlabeled cells, fluorescence quenching, limitation, etc., and achieve the effects of excellent luminescence performance, short synthesis route, and strong practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: (R in general formula 1 does not exist)
[0034]
[0035] (1) in N 2 7.18g of zinc powder was added to 80mL of dry THF, and stirred at 0°C for 10mins. In an ice bath, use a syringe to dispense 6 mL of TiCl 4 Slowly added dropwise to the reaction, heated to reflux for 2h. Then, 5 g of benzophenone (compound 1) was dissolved in 20 mL of THF, slowly added dropwise using a constant pressure dropping funnel, and heated to reflux for 12 h. After the reaction, use 10% K 2 CO 3 The solution was quenched and extracted with ethyl acetate, the organic phase was washed with water, the organic phase was collected and washed with anhydrous Mg 2 SO 4 dry. Finally, the solvent of the organic phase was removed using a rotary evaporator, and the obtained product was washed with ethanol to finally obtain 3.8 g of white solid tetraphenylethylene.
[0036] (2) Dissolve tetraphenylethylene (compound 2) obtained in (1) in CH 2 Cl 2 , and slowly add 3mL of Br 2 , s...
Embodiment 2
[0041] Embodiment 2: (R in general formula 1 is a double bond for the parent nucleus)
[0042]
[0043] (1) in N2 Next, compound 3, 42 mg of palladium acetate, 200 g of K 2 CO 3 and 44mg of tricyclohexylphosphine were added to the reaction tube, then 10mL of DMF and 130g of 4-vinylpyridine were added, heated to reflux for 48h, and then cooled to room temperature. The solvent was removed by rotary evaporator with CHCl 3 The product was extracted and washed with water, the organic phase was collected and washed with anhydrous Mg 2 SO 4 dry. Finally, the organic phase was purified by silica gel column chromatography, the eluent was CHCl3:MeOH=30:1, and the yellow product 6 was finally obtained.
[0044] (2) Compound 6 with 29mg BrCH 2 CH 2 COOH was dissolved in 1mL DMF and heated to reflux for 24h. Then, cool to room temperature, add excess acetone, collect the precipitate, wash the precipitate with acetone and petroleum ether, and dry in vacuum to obtain the final co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com